Chapter 9 Reproductive physiology
9.1 Sexual development
Puberty
The trigger for the onset of puberty is unknown, but some change affecting the hypothalamus seems to be crucial. In both sexes, one of the first detectable developments is an increase in the plasma concentrations of the gonadotrophins, i.e., follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These are protein hormones secreted by the anterior pituitary gland, but their secretion is controlled by gonadotrophin-releasing hormone (GnRH), a peptide secreted by the hypothalamus (Section 8.2). Why GnRH secretion should rise at puberty is not known, but the resulting increase in gonadotrophin levels stimulates the previously dormant testes or ovaries. This initiates spermatogenesis in the male and cyclical follicular development and ovulation in the female. At the same time, the endocrine activity of the gonads causes an increase in the levels of the sex steroids—testosterone in the male and oestrogens in the female—promoting the secondary sex characteristics.
9.2 Male reproductive function
Relevant structure
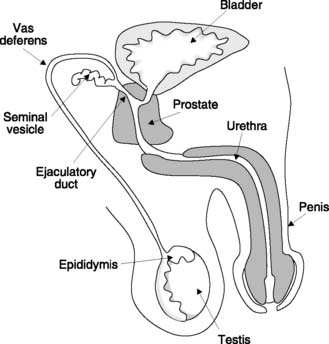
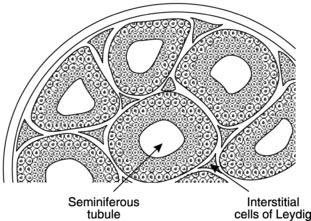
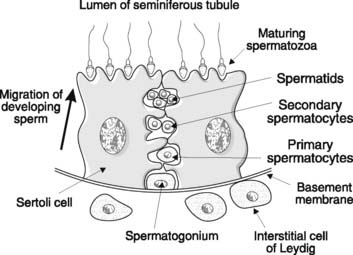
The male gonad is the testis (Fig. 167). This is the source of the male gametes, the spermatozoa, and produces the male sex hormone testosterone. These functions rely on two different structures in the testis. Spermatogenesis occurs in the seminiferous tubules, while testosterone is secreted by specialized cells in the interstitium between these tubules known as the interstitial cells of Leydig (Fig. 168).
During the last months of fetal development, the testes descend through the inguinal canals from their initial position in the body cavity, so that at birth they lie within the scrotum. Like other aspects of male sexual development, this migration is stimulated by fetal testosterone. Normal sperm production (spermatogenesis) only occurs 2–3 °C below body core temperature, and if a testis fails to descend into the scrotal sac it will not function properly. Testicular temperature can actually be regulated using the cremaster muscle around the spermatic cord and the dartos muscle in the wall of the scrotum. In cold weather these contract, pulling the testis and scrotum towards the body and thus conserving heat.
Once structurally mature, spermatozoa are flushed into the epididymis which encloses the posterior pole of the testis (Fig. 167). Removal of fluid leaves a condensed mass of sperm which reach functional maturity within the epididymis, becoming motile. They then move on into the vas deferens, a muscular tube which acts as a storage reservoir for sperm. At its distal end, the vas fuses with the duct from the seminal vesicle, a reproductive exocrine gland, to form the ejaculatory duct. This passes through the prostate gland at the base of the bladder and several ducts from the prostate empty into the ejaculatory duct before it opens into the urethra.
Spermatogenesis
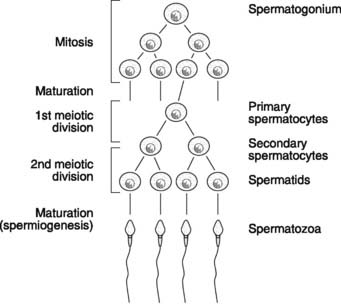
Sperm production continues throughout life from puberty onwards. The development of spermatozoa from spermatogonia, which are distributed around the periphery of the seminiferous tubules in the testes, is called spermatogenesis (Fig. 169). Spermatogonia first divide mitotically and then mature into primary (1 °) spermatocytes. Since only some of the cells resulting from division develop further, however, leaving others in reserve for future replication, the total number of spermatogonia is not depleted. This allows continuous production of gametes from puberty onwards, and contrasts with oogenesis in the female, in which no new primary oocytes are formed after birth (Section 9.4).
Next, the primary spermatocytes (diploid cells containing 23 homologous chromosome pairs) undergo meiosis (Fig. 169). The DNA is replicated prior to the first meiotic division, with each chromosome forming two sister chromatids. There is then exchange of genetic material (crossing over) between the partner chromosomes in each chromosome pair, and this leads to new gene combinations in the daughter cells. Separation of the chromosome pairs followed by cell division completes the first meiotic division, producing two secondary (2 °) spermatocytes. Each of these contains half the normal complement of chromosomes (one chromosome from each pair), i.e., these are haploid cells. It is important to realize, however, that each chromosome still consists of two connected sister chromatids, so 2 ° spermatocytes actually contain the normal number of genes. There is no further replication of DNA prior to the second meiotic division, however, during which the chromatids separate. This produces four haploid spermatids, each containing one chromosome from each chromosome pair and one gene from each gene pair.
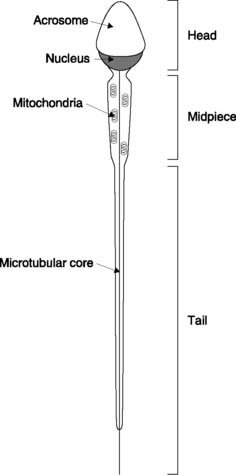
There is no further cell division, but the spermatids must still undergo a final maturation process to produce spermatozoa. This is sometimes referred to as spermiogenesis and results in the head, midpiece and tail structures of the mature spermatozoon (Fig. 170). The head consists of the chromosomal material covered by an enzyme-filled sac called the acrosome. The midpiece contains mitochondria, which provide the ATP necessary for sperm motility. This motility is the result of the swimming action of the tail, which is driven by the contractile activity of its microtubular core.
Sertoli cell function
At each stage of spermatogenesis, there is a close association between the developing sperm and large cells which line the seminiferous tubules, known as Sertoli cells (Fig. 171). These fulfil the following functions:
Hormonal control of male reproduction
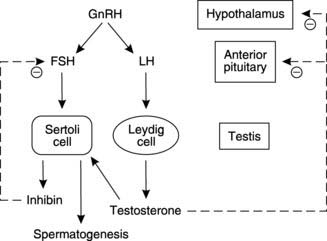
Control of male reproductive function depends on a series of hormones whose secretion is regulated through negative feedback loops (Fig. 172). The overall pattern has several similarities to that seen in control of the female reproductive system (see Fig. 176). The hypothalamus produces GnRH which is carried in the hypophyseal portal system to the anterior pituitary (Section 8.2). This stimulates release of the gonadotrophins, FSH and LH, which are carried in the circulation to the testes, their site of action. Each acts on a different target cell, with FSH stimulating spermatogenesis through its action on the Sertoli cell, while LH promotes testosterone secretion from the interstitial cells of Leydig. This results in a high tissue concentration of testosterone in the testis, which is necessary for spermatogenesis. Circulating testosterone exerts a negative feedback effect by inhibiting hypothalamic and anterior pituitary secretion (Fig. 172). Sertoli cells also secrete a peptide hormone called inhibin, which has a relatively specific effect in reducing FSH secretion.
9.3 Sexual intercourse
Sexual intercourse makes fertilization possible by introducing sperm to the female genital tract. It involves erection of the penis, allowing penetration of the vagina to occur, followed by ejaculation of semen, a mixture of sperm and secretions from the glands of the male reproductive tract.
Ejaculation
Ejaculation is a two-stage process.
9.4 Female reproductive function
Relevant structure
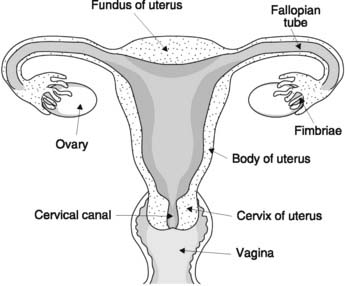
The female gonad is the ovary which produces ova and secretes the female sex steroids, i.e., oestrogens (particularly estradiol) and progesterone. The ovaries are loosely enfolded in the frond-like fimbriae which guide ova into the Fallopian, or uterine, tubes (Fig. 173). These are connected to the uterus, which opens through the cervix into the vagina below.
Oogenesis
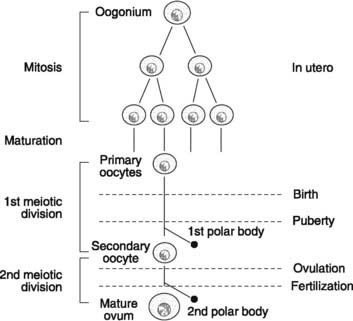
Oogenesis refers to the development of mature ova from their primitive precursors, the oogonia (Fig. 174). Initially, the oogonia multiply by mitotic division and then undergo a maturation process to form the primary oocytes within the ovary. Multiplication only occurs during fetal life, however, and the 1–2 × 106 primary oocytes present at birth are the source for all the mature ova which will develop during reproductive life. This should be contrasted with the continuous replication of spermatogonia in postpubertal males. Only 400–500 primary oocytes ever reach maturity, while the rest simply degenerate.
The primary oocytes (diploid cells) begin, but do not complete, the first meiotic division before birth (Fig. 174). All further development is arrested until after puberty, when the first meiotic division may finally be concluded. This produces a secondary oocyte (a haploid cell), which contains nearly all the available cytoplasm and a small polar body, which consists largely of chromosomal material. Formation of the secondary oocyte is delayed until just prior to ovulation, and the secondary oocyte only completes the second meiotic division if it is fertilized. Under these circumstances, a further uneven division of cell resources occurs, producing the mature ovum (containing 22 autosomes and a single sex chromosome) and a second polar body.
The female reproductive cycle
Ovarian cycle and follicular development
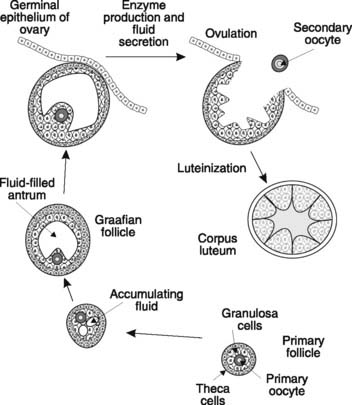
Ova develop within structures known as follicles. Primordial follicles form in utero and consist of a primary oocyte surrounded by the basal lamina and a single layer of granulosa cells. At any time from late intrauterine life onwards, primordial follicles may mature further to form primary follicles. Granulosa cells multiply and lay down a jelly-like, mucopolysaccharide layer, the zona pellucida, around the oocyte, and a layer of cells outside the basal lamina differentiates to form theca cells (Fig. 175).
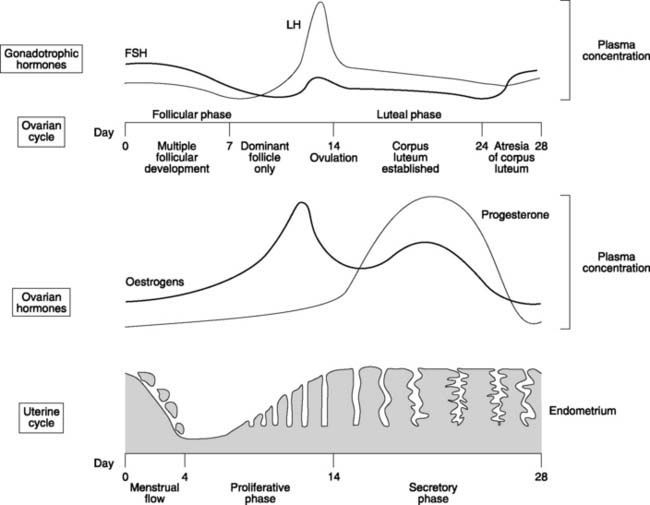
Although many primary follicles simply regress during life, a proportion will go on to develop further during the ovarian cycles of reproductive life. These cycles commence at puberty and each cycle is normally 28 days long, consisting of a 14-day follicular phase followed by ovulation and a 14-day luteal phase.
Follicular phase
From puberty onwards, about 5–15 primary follicles begin to develop at the start of each reproductive cycle. Granulosa cells multiply and secrete fluid which eventually pools to form the antrum, thus producing an antral or Graafian follicle (Fig. 175). The theca cells also multiply and differentiate to form the theca interna surrounded by the theca externa. After 7–10 days, one follicle becomes dominant and enlarges further, while the rest become atretic. By 14 days, the developing follicle actually distorts the ovarian surface. Rapid fluid accumulation increases the intrafollicular pressure and proteolytic enzymes digest the overlying tissues. Ovulation follows as the follicle ruptures, releasing the ovum (a secondary oocyte) surrounded by the zona pellucida and adherent granulosa cells (the corona radiata) into the Fallopian tube (see Fig. 178). Some women report that they can feel the rupture of the follicle, which may be painful.
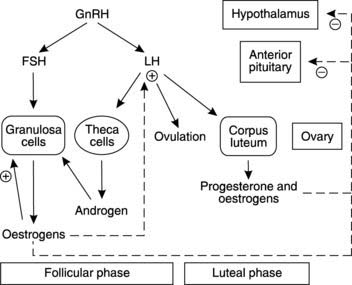
Hormonal control of the ovarian cycle
This is similar to control of male reproduction (Fig. 172), except for the monthly cycling of hormone levels seen in women. As in males, the hypothalamus produces GnRH, which causes release of the gonadotrophins FSH and LH from the anterior pituitary gland (Fig. 176). As its name suggests, FSH stimulates follicular development by activating granulosa cell division and secretion of oestrogens (e.g., estradiol). During the follicular phase, LH plays a supporting role as it stimulates androgen secretion by the theca cells. These androgens diffuse into the granulosa cells, which use them as precursors for the synthesis of oestrogens. LH is also responsible for triggering ovulation once follicular development is complete, and it then stimulates luteinization of the follicular remnants to form the corpus luteum. This secretes both progesterone and oestrogen.
Using the ideas outlined above, we can attempt to explain the normal pattern of hormonal changes seen during a single, 28-day, reproductive cycle (Fig. 177). At the beginning of the cycle, oestrogen and progesterone levels are low, so they exert little negative feedback and FSH levels are high. This stimulates follicular development, leading to a rise in oestrogen levels with resultant inhibition of gonadotrophin release. FSH and LH concentrations fall to a minimum about day 10–12. At this stage, the very high oestrogen concentrations appear to generate a positive feedback effect, however, leading to a dramatic peak in LH concentrations. This LH surge stimulates ovulation, which occurs about day 14. Thereafter, increasing production of progesterone and oestrogen by the corpus luteum leads to a steady decline in LH and FSH. At about day 22–24, the corpus luteum undergoes programmed regression, or atresia, causing a rapid decline in the levels of ovarian hormone. The resulting reduction in feedback inhibition allows gonadotrophin levels to rise once more, and the cycle repeats.
Female sex hormones
Oestrogens are a group of related chemicals. Oestradiol and oestrone make up most of the circulating oestrogen in the non-pregnant state during the reproductive years, but oestrone levels exceed estradiol postmenopausally. Placental oestriol becomes important during pregnancy.
The uterine cycle
The endometrium consists of a mixture of glands and blood vessels within a connective tissue stroma. At the beginning of each cycle, approximately two-thirds of the endometrial thickness sloughs off from the underlying endometrium and myometrium and is lost during menstruation (Fig. 177). This lasts 2–5 days. Rising oestrogen levels then stimulate hyperplasia of the endometrium, with the growth of many simple tube-like glands. This is the proliferative phase of the uterine cycle. Following ovulation, progesterone becomes the dominant sex hormone and this causes the glands to become tortuous, or saw-toothed, in outline. This is referred to as the secretory phase because the glands also fill up with secretions rich in glycogen. The purpose of this is to provide a highly vascular, fluid- and nutrient-rich environment suitable for implantation of a developing embryo. If no pregnancy occurs, the rapid fall in oestrogen and progesterone levels following atresia of the corpus luteum around day 24 causes vascular constriction, ischaemia and infarction of the endometrium, which is shed in the next menstrual flow.
9.5 Pregnancy, labour and lactation
Fertilization
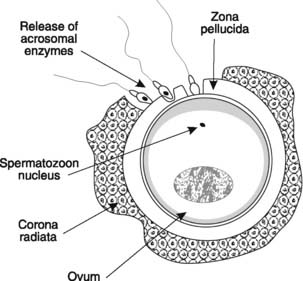
For fertilization to occur, spermatozoa and ovum must first come into contact with each other. Following ovulation, the ovum is propelled by the movement of the cilia lining the fimbriae and Fallopian tube. Progress is rather slow relative to the upward migration of the sperm through the female genital tract, and fertilization usually occurs in the outer third of the Fallopian tube. The spermatozoa are deposited in the vagina during sexual intercourse and first have to pass through the mucus which plugs the cervical canal (Fig. 173). This is only penetrable towards the end of the follicular phase of the ovarian cycle, around ovulation, when the ratio of oestrogen to progesterone is at its peak (Fig. 177). For a few days the mucus plug becomes much less dense, allowing sperm to swim through it, but even then less than 5% actually enter the uterus. Further transport depends on the contractile activity of the uterus, which randomly distributes sperm throughout the uterine cavity. Some enter the Fallopian tube and are propelled towards the ovum by retrograde peristalsis. Of as many as 300 × 106 spermatozoa released during ejaculation, perhaps only a few thousand are likely to reach the ovum. This explains the need for high sperm counts if a male is to be fertile. It should also be noted that the fertilizing ability of sperm is enhanced following several hours exposure to the female reproductive tract, a process referred to as capacitation.
Fertilization requires that a spermatozoon must first penetrate the corona radiata and the zona pellucida which surround the ovum (Fig. 178). The motility of the sperm orients their heads towards these barriers and forces them between the granulosa cells of the corona radiata. They then bind to receptors on the outer surface of the zona pellucida and digest a tunnel through it by releasing acrosomal enzymes. When the first spermatozoon contacts the plasma membrane of the secondary oocyte, the zona pellucida changes chemically, becoming impenetrable to other sperm. This prevents multiple fertilizations of the same ovum. The secondary oocyte completes the second meiotic division and the genetic material from the haploid gametes fuses to produce a diploid zygote. This then divides, initiating embryonic development.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree