Renal Artery Intervention for Atherosclerotic Occlusive Disease
Kimberley J. Hansen
K. Todd Piercy
With the introduction of new antihypertensive agents specific to the renal angiotensin system and percutaneous renal artery angioplasty (PTAS) with endoluminal stenting, attitudes regarding open surgical intervention for atherosclerotic renovascular disease have changed. These new treatment alternatives have led many physicians to limit open surgical intervention to patients with (a) severe hypertension despite maximal medical therapy, (b) anatomic failures or disease patterns not amenable to balloon angioplasty and stenting, or (c) renovascular disease complicated by renal excretory insufficiency (i.e., ischemic nephropathy). Our strategy for open operative management of atherosclerotic renovascular disease is outlined as follows:
Severe hypertension is considered a prerequisite for surgical intervention. Prophylactic repair of clinically silent, occlusive renal artery disease is not advised.
All clinically significant renal artery disease (i.e., associated with severe hypertension and/or excretory renal insufficiency) is repaired at a single operation. The exception to this approach is the individual who requires bilateral ex vivo renal reconstruction. In these cases, ex vivo operations are staged.
Direct aortorenal reconstruction is favored over indirect or splanchnorenal repair. Celiac axis disease accompanies renal artery disease in 40% of patients. Up to 60% of patients require bilateral renal artery reconstruction and 30% require combined aortic repair.
Nephrectomy is performed infrequently. Nephrectomy is reserved for treatment of an unreconstructable renal artery lesion to a nonfunctioning kidney contributing to severe hypertension. Renal artery occlusions are reconstructed when a normal distal renal artery is present.
Simultaneous aortic repair is combined with renal artery reconstruction only when aortic disease is clinically severe. Aortic repair is not undertaken to provide an inflow source for renal reconstruction.
Regardless of the technique of reconstruction, each repair is assessed with intraoperative renal duplex sonography. Major defects requiring immediate revision are found in 10% to 12% of arterial repairs.
Patients who require multiple medications for hypertension may experience reduced medication requirements while hospitalized at bed rest. In this instance, hypertensive medications are reduced to minimum levels necessary for blood pressure control prior to operative repair. Both converting enzyme inhibitors and angiotensin receptor antagonists are discontinued. Preoperative and postoperative medical therapy for blood pressure control includes vasodilators (e.g., amlodipine, nicardipine) and selective β-adrenergic blocking agents (e.g., atenolol, metoprolol). Patients with severe hypertension (i.e., diastolic pressures exceeding 110 mm Hg) require postponement of operative treatment until pressure is brought under control. A combination of intravenous sodium nitroprusside and esmolol administered in an intensive care unit with continuous intra-arterial blood pressure monitoring may be required. Patients with significant heart disease, in combination with severe renal insufficiency, may require preoperative placement of invasive monitoring to optimize cardiac index and intravascular volume prior to operation.
Certain steps are common to almost every open renal artery procedure. Intravenous mannitol is administered during periods of aortic and perirenal dissection as well as before and after periods of renal ischemia. Small repeated intravenous doses are administered up to a total dose of 1 g/kg body weight. Prior to aortic or renal artery cross clamp, 100 units of heparin per kilogram of body weight are administered intravenously and systemic anticoagulation is verified by activated clotting time. During periods aortic or renal cross clamp, measurement of activated clotting time is repeated every 45 minutes and additional intravenous heparin administered as necessary. Protamine is not routinely administered at the conclusion of procedure unless required for hemostasis.
Renal Artery Exposure
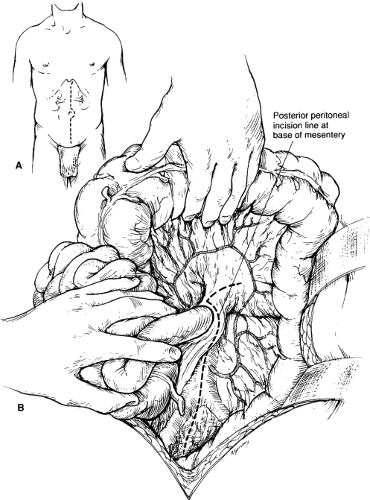
Open repair of atherosclerotic renal artery disease is most commonly made through a midline xiphoid to pubis abdominal incision. The patient is positioned with the operating table flexed. Extending the proximal incision 1 to 2 cm to one side of the xiphoid is required to obtain full exposure of the upper abdominal aorta and renal arteries (Fig. 1A). A fixed mechanical retraction system is advantageous, especially when combined aortorenal reconstruction is performed. Extended flank incisions coursing from the contralateral semilunar line and bisecting the ipsilateral costal margin and pelvic crest are useful for combined visceral–renal artery reconstruction and ex vivo branch renal artery repair. When performed
from an extended left flank incision, supraceliac exposure of the aorta is facilitated. In this instance, the ipsilateral flank is elevated on rolled sheets and the left arm is padded and positioned posteriorly. A left visceral mobilization provides access to the renal vasculature, the celiac axis, the superior mesenteric artery, and the supraceliac aorta. If required, the aortic crura can be divided, allowing for extrapleural aortic dissection to the T10 level of the descending thoracic aorta for proximal control and reconstruction.
from an extended left flank incision, supraceliac exposure of the aorta is facilitated. In this instance, the ipsilateral flank is elevated on rolled sheets and the left arm is padded and positioned posteriorly. A left visceral mobilization provides access to the renal vasculature, the celiac axis, the superior mesenteric artery, and the supraceliac aorta. If required, the aortic crura can be divided, allowing for extrapleural aortic dissection to the T10 level of the descending thoracic aorta for proximal control and reconstruction.
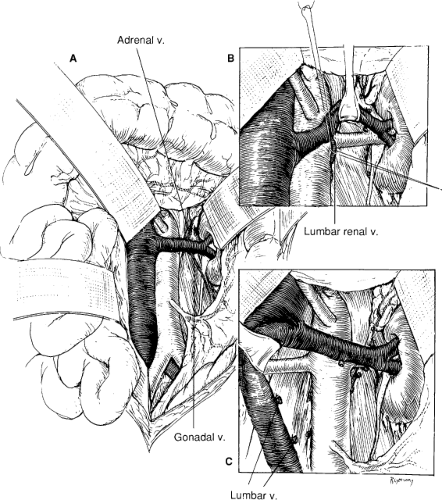
When a midline abdominal incision is performed, the posterior peritoneum overlying the aorta is incised longitudinally (Fig. 1B). The ligament of Treitz is mobilized with care to identify visceral collaterals, which may course with the inferior mesenteric vein at this level (Fig. 1B). The duodenum is reflected to the patient’s right to expose the renal vein, which is mobilized from the vena cava origin to the renal hilum (Fig. 2A). By so doing, the inferior border of the pancreas is also mobilized in an avascular plane, allowing exposure of the left renal hilum. Depending on the course of the left renal artery, the gonadal, adrenal, and lumbar renal branches of the left renal vein may be divided to retract the vein either superiorly or inferiorly (Fig. 2B). The proximal portion of the right renal artery can be exposed by this same approach. The entire retrocaval portion of the right renal artery can be exposed by ligating one or more pairs of lumbar veins (Fig. 2C).
When distal exposure of the renal artery is required or unilateral branch renal artery repair is planned, unilateral flank incision may be preferred. The right renal artery exposure is achieved by mobilization of the hepatic flexure of the colon combined with mobilization of the duodenum with an extensive Kocher maneuver (Fig. 3). An avascular plane is entered anterior to the kidney, sweeping the duodenum medially (Fig. 4A). The vena cava is identified and the origin of the right renal vein is noted. The right renal artery usually courses posterior to the vein, which may be retracted either superiorly or inferiorly to provide the best exposure. Supernumerary renal arteries occur on both the right and left in 15% to 20% of patients. All arteries coursing anterior to the vena cava should be considered supernumerary or polar renal arteries to the right kidney and carefully preserved (Fig. 4B).
Aortic exposure proximal to the renal arteries is facilitated by partial division of the diaphragmatic crura. This maneuver allows exposure of the aorta proximal to origin of the superior mesenteric artery. Exposure and control of the aorta at this level is required during transaortic thromboendarterectomy.
Open Renal Artery Reconstruction
No single method of open renal artery repair provides optimal reconstruction for all renal artery lesions. The method that best conforms to the patient and the disease process must be selected. However, of the three basic methods of renal artery repair (i.e., aortorenal bypass, transaortic thromboendarterectomy, and renal artery reimplantation), renal artery bypass is the most versatile. Saphenous vein is the preferred conduit for an isolated renal artery bypass in an adult. Hypogastric artery is selected for renal reconstruction in the child and adolescent. In the absence of satisfactory autogenous material and a renal artery of at least 4 mm in diameter, 6-mm thin-walled polytetrafluoroethylene provides patency equivalent to autogenous reconstruction.
Aortorenal Bypass
Regardless of the material chosen for aortorenal bypass, proximal aortorenal anastomosis is performed first. A segment of in-frarenal aorta proximal to the inferior mesenteric artery is controlled and an aortotomy created along the anterolateral aspect of the aorta. It is important to excise
an ellipse of aorta with the length of the aortotomy at least three times the diameter of the renal artery conduit. Several applications of a 4.8-mm aortic punch can be used to create this ellipse. The proximal anastomosis is performed with a continuous 6-0 polypropylene suture (Fig. 5).
an ellipse of aorta with the length of the aortotomy at least three times the diameter of the renal artery conduit. Several applications of a 4.8-mm aortic punch can be used to create this ellipse. The proximal anastomosis is performed with a continuous 6-0 polypropylene suture (Fig. 5).
In the past, the distal graft to renal artery anastomoses was frequently created in an end-to-side fashion; however, distal end-to-end anastomosis is preferred. In this case, both conduit and renal artery are spatulated widely (two to three times the diameter of the renal artery) and the anastomosis accomplished with a continuous 7-0 polypropylene suture. Prior to the completion of the anastomosis, the graft is flushed of air and debris and blood flow reestablished to the kidney. In most instances, the warm renal ischemia time is 20 minutes or less.
Thromboendarterectomy
Transaortic thromboendarterectomy is particularly useful in patients with multiple renal arteries that demonstrate orificial atherosclerotic stenosis. Prior to undertaking thromboendarterectomy, the extent of atherosclerotic disease should be clearly defined. All visible and palpable renal artery atheroma should end within 1 to 1.5 cm of the aortic origin.
Thromboendarterectomy requires more extensive aortic exposure than aortorenal bypass (Fig. 6). The aorta proximal to the superior mesenteric artery should be exposed for control at this level (Fig. 6B). As discussed previously, this is facilitated by partial division of the diaphragmatic crura. The superior mesenteric artery origin is exposed and controlled with an elastic loop. After preliminary control of each renal artery and after proximal and distal aortic control, the aorta is opened longitudinally to the base of the superior mesenteric artery (Fig. 6A). First a sleeve endarterectomy of the aorta is performed. The correct endarterectomy plane is best defined at the site of the most advanced atherosclerotic disease. The aortic atheroma is divided sharply at the proximal and distal end points flush with the residual adventitia. Distal tacking sutures may be applied if necessary. Following sleeve endarterectomy of the aorta, an eversion type of endarterectomy of each renal artery is performed. The renal artery is everted into the aorta to allow the distal end point of the endarterectomy to be visualized (Fig. 6B). The aortic arteriotomy is then closed primarily with a continuous 5-0 polypropylene suture.
As with thromboendarterectomy at all anatomic sites, this procedure is contraindicated by the presence of degenerative aneurysmal change of the aortic wall and aortic atheroma complicated by transmural calcification. This later condition is defined by gentle palpation of the aorta. Transmural calcification may feel like fine-grade sandpaper on palpation. When thromboendarterectomy is performed in this setting, multiple defects may be created within the adventitia, which are apparent only after blood flow is restored.
Renal Artery Reimplantation
After extensive mobilization of the renal artery, there may be sufficient redundancy of the vessel to allow for aortic reimplantation. As with aortorenal bypass, the procedure requires that an ellipse of the aorta be taken and that the renal artery be spatulated two to three times its diameter (Fig. 7B,C). Renal artery reimplantation has particular application to children and adolescents with hypoplastic orificial lesions. The technique obviates concerns regarding renal artery conduit in these age groups.
Splanchnorenal Bypass
Splanchnorenal bypass has received increased use as an alternative method for renal revascularization. It is essential that the celiac axis and its branches be free of hemodynamically significant disease prior to reconstruction. In addition, significant compression during respiration by arcuate ligament must be excluded.
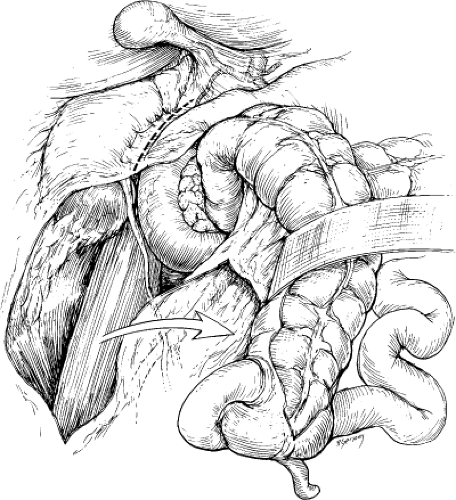 Fig. 3. With the right colon mobilized medially, a Kocher maneuver exposes the right renal hilum. (From Benjamin ME, Dean RH. Techniques in renal artery reconstruction: part I. Ann Vasc Surg 1996;10:409, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|