Optional Tests
• Maximal urinary flow rate. It is tested if greater than 15 mL per second excludes clinically important BOO due to BPH.
• Measurement of postvoid residual urine (PVR) by in-and-out catheterization, ultrasound, or cystography. Large PVR correlates with severe BPH and chronic renal insufficiency, but does not predict need for surgery. Normal men have less than 12 mL of residual urine.7
• Urine cytology. Urine cytology may be helpful in men with predominantly irritative symptoms. It may be considered in men with a smoking history, since this is a risk factor for bladder cancer.6
• Newer technologies. It is also possible that newer imaging modalities, such as contrast-enhanced MRI and MR diffusion, will be able to differentiate glandular-ductal versus stromal-low ductal tissues.8 Such information may aid in the detection of cancer and its grading. It is still unclear whether this information will prove to be cost-effective.
Differential Diagnosis
The differential includes urethral stricture (history of urethral trauma, urethritis, urethral instrumentation), bladder neck obstruction, carcinoma of the prostate or urinary bladder (hematuria, persisting LUTS not responding to treatment, hard nodular prostate on DRE, elevated PSA), and urolithiasis (pain, hematuria).
• Other considerations include lower urinary tract infections (UTIs), including prostatitis (frequency, urgency, abdominal pain, perirectal pain, constitutional symptoms, boggy and tender prostate) and neurogenic bladder (history of cerebral or spinal injury or tumor, stroke, degenerative joint disease of the spine, spinal or pelvic surgery; urinary retention or incontinence; focal neurologic deficits on examination; cystoscopy and pressure flow studies are useful).6
TREATMENT OPTIONS
Watchful Waiting (Active Surveillance)
• Appropriate for patients with mild symptoms (symptom score less than 7) and minimally enlarged prostate.4
• Medications exacerbating symptoms or inducing urinary retention (sedating antihistamines, decongestants) should be avoided.
• Voiding at regular intervals, pelvic floor exercises, and avoidance or treatment of constipation are recommended.
• Fluid intake should be minimized before bedtime; caffeine and alcohol intake are reduced.
Medication
Indicated when symptoms of BPH impact the patient’s quality of life in spite of implementing the above treatment or AUA-SI score ≥8.4 Two classes of drugs, α-adrenergic antagonists and 5-α-reductase inhibitors (5-ARIs), act upon the dynamic (tension of prostatic smooth muscle in the prostate, prostate capsule, and bladder neck) and fixed (the bulk of the enlarged prostate impinging upon the urethra) components of BOO, respectively.9
• α-Blockers
• Smooth muscles in the prostate gland contract in response to α-adrenergic receptor stimulation, causing constriction of the prostatic urethra. α1-Receptor antagonists improve LUTS by promoting smooth muscle relaxation.
• FDA has approved terazosin, doxazosin, tamsulosin, alfuzosin, and silodosin for the treatment of the symptoms of BPH.9
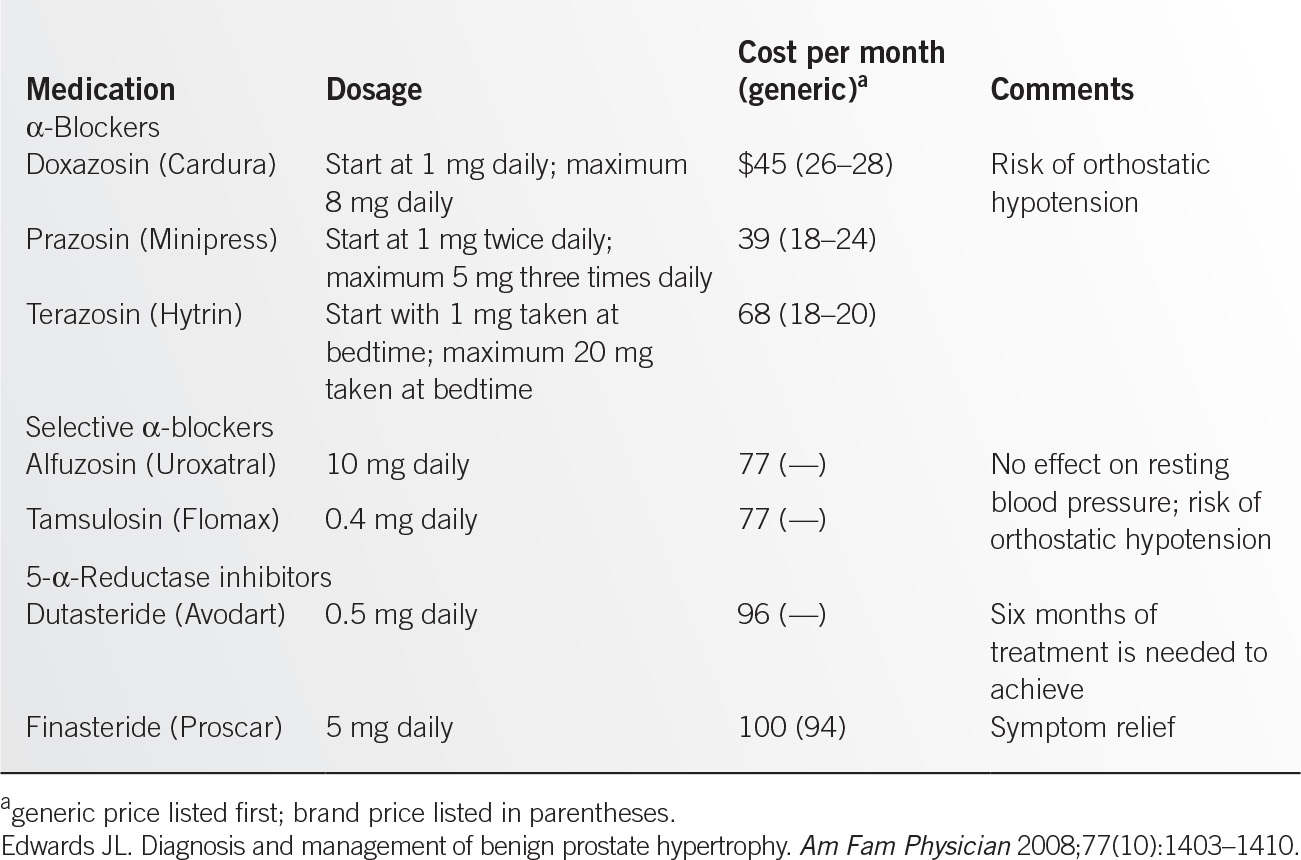
![]() Dosage and adverse effects (Table 12.5-2).
Dosage and adverse effects (Table 12.5-2).
• These are considered the most effective monotherapy for improving LUTS in men with BPH.4
• Symptom improvement is typically noted within 2 to 4 weeks of initiating α-blocker therapy.1
• Side effects: Most important are orthostatic hypotension and dizziness. Terazosin and doxazosin need to be initiated at bedtime, and dose needs to be titrated up over several weeks.9
• 5-ARIs
• These act upon the fixed component of prostate by reducing its size. 5-α-Reductase blockers inhibit the conversion of testosterone to dihydrotestosterone, suppressing prostate growth.10
• FDA has approved finasteride and duasteride for the treatment of BPH.
• For dosage and adverse effects, see Table 12.5-2.
• These have been shown to affect the clinical course of BPH, reducing the risk of acute urinary retention (NNT = 26) and surgical intervention (NNT = 18) 4 years after therapy.11
• It usually takes 6 to 12 months in order to see any symptom relief.
• Combination therapy
• is an appropriate and effective treatment for patients with LUTS associated with demonstrable prostatic enlargement based on volume measurement, PSA level as a proxy for volume, and/or enlargement on DRE.4
• Alternative therapies
• Saw Palmetto
• Rye grass pollen extract (Cernilton)
• Pygeum
![]() AUA does not recommend for treatment of LUTS related to BPH4
AUA does not recommend for treatment of LUTS related to BPH4
Nonsurgical Treatment (Minimally Invasive Treatment)
Useful in those with significant comorbid disease, and in patients requiring chronic anticoagulation.4
• Transurethral needle ablation of the prostate (TUNA). Low-energy radiofrequency ablation performed under local anesthesia, which improves symptom scores and urinary flow rates in 50% to 60% of patients, with minimal complications.4
• Transurethral microwave thermotherapy (TUMT). It involves heating prostatic tissue using computer-regulated microwaves under local anesthesia. Higher energy TUMT is useful in patients with larger glands. It improves symptom scores and urinary flow. Serious thermal injuries may result.4
Surgical Treatment
The AUA recommends surgery in patients with renal insufficiency secondary to BPH, recurrent UTIs, bladder stones or gross hematuria due to BPH, acute urinary retention, or those who have LUTS refractory to other therapies.4
• Laser therapies. Transurethral holmium laser ablation of the prostate (HoLAP), transurethral holmium laser enucleation of the prostate (HoLEP), and holmium laser resection of the prostate (HoLRP) are effective treatment alternatives to transurethral resection of the prostate and open prostatectomy in men with moderate to severe LUTS and/or those who are significantly bothered by these symptoms.4
• Transurethral incision of the prostate (TUIP). Making two deep incisions distal to each ureteral orifice through the bladder neck and the prostatic adenoma toward the verumontanum, down to the capsule of the prostate. It is recommended for men with BOO and minimal prostate enlargement, especially those with comorbid illnesses.4
• Open prostatectomy. This procedure is performed infrequently (in under 5%) on patients with large prostates, who are good surgical candidates. Retropubic, transvesical, and perineal approaches exist.4
• Newer treatment. In September 2013, the FDA approved Urolift, the first permanent implant to relieve low or blocked urine flow in men aged 50 years and older with an enlarged prostate.12 It relieves the urine flow by pulling back the prostate tissue that is pressing on the urethra.12
Special Considerations: Indications for Referral to a Urologist
• History of worsening LUTS, acute urinary retention, hematuria, urinary incontinence without response to conservative and medical treatment
• Palpable urinary bladder or high PVR
• Urolithiasis, recurrent UTIs
• Hard and irregular prostate, elevated PSA9
ACKNOWLEDGMENT
The author would like to acknowledge Kalyanakrishnan Ramakrishnan for his work on the previous version of this chapter.
REFERENCES
1. Edwards JL. Diagnosis and management of benign prostatic hyperplasia. Am Fam Physician 2008;77(10):1403–1410.
2. Deters LA, Kim ED. Benign prostatic hypertrophy. Medscape. http://emedicine.medscape.com/article/437359-overview#a0101. Accessed November 3, 2013.
3. Cunningam GR, Cadmon D. Epidemiology and pathogenesis of benign prostatic hyperplasia. UptoDate. www.uptodate.com. Accessed November 2, 2013.
4. American Urological Association. Guideline on the management of benign prostatic hyperplasia (BPH). http://www.auanet.org/common/pdf/education/clinical-guidance/Benign-Prostatic-Hyperplasia.pdf. Accessed November 3, 2013.
5. Parsons JK, Carter HB, Partin AW, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab 2006;91:2562.
6. Cunningam GR, Cadmon D. Clinical manifestations and diagnostic evaluation of benign prostatic hyperplasia. UptoDate. www.uptodate.com. Accessed November 2, 2013.
7. DiMare JR, Fish SR, Harper JM, et al. Residual urine in normal male subjects. J Urol 1963;96:180.
8. Noworolski SM, Vigneron DB, Chen AP, et al. Dynamic contrast-enhanced MRI and MR diffusion imaging to distinguish between glandular and stromal prostatic tissues. Magn Reson Imaging 2008;26:1071.
9. Cunningam GR, Cadmon D. Medical treatment of benign prostatic hyperplasia. UptoDate. www.uptodate.com. Accessed November 2, 2013.
10. McNaughton-Collins M, Barry MJ. Managing patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Am J Med 2005;118(12):1331–1339.
11. McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998;338(9):557–563.
12. Crane M. FDA OKs New Device to Treat BPH. Medscape. medscape.com/viewarticle/810981. Accessed November 3, 2013.
|
GENERAL PRINCIPLES
Prostate cancer is the most common noncutaneous cancer in men in the United States. An estimated one in six white men and one in five African American men will be diagnosed with prostate cancer in their lifetime, with the likelihood increasing with age. It is the second most common cause of cancer death in males.1
Anatomy
Prostate cancer usually arises in the peripheral zone of the gland, but it may also occur laterally or centrally and is often multifocal. If it grows, it may become locally invasive—penetrating the prostate capsule, invading the seminal vesicles, spreading to pelvic lymph nodes, and eventually reaching distant lymph nodes in the abdomen and beyond. Bone is usually the first site of metastasis beyond the lymph nodes, with liver and lung metastases occurring later.2
Epidemiology
The American Cancer Society estimates that there will be 238,590 new cases of prostate cancer diagnosed in 2013 and 29,700 deaths. Incidence increases after age 50, with more than two thirds of prostate cancers diagnosed after the age of 65.3 Possessing certain genes increases the risk of prostate cancer. History of prostate cancer in a brother or father doubles the risk of the disease. African American men have a 60% higher incidence of prostate cancer compared to whites, whereas Asian and Hispanic Americans have lower rates than whites. African American men are also more often diagnosed with advanced disease and twice as likely as other American men to die of prostate cancer. Since the early 1990s, prostate cancer screening has dramatically increased the number of prostate cancers diagnosed in the United States, with a simultaneous shift toward earlier stage at diagnosis. Meanwhile, prostate cancer deaths declined. However, depending on the prostate-specific antigen (PSA) value, pathologic stage, and histologic grade of the tumor, approximately 30% of patients with clinically localized prostate cancer are estimated to progress despite initial treatment with intent to cure.4
Pathophysiology
Prostate cancer develops when the rates of cell division and cell death are unequal, leading to uncontrolled tumor growth. Further mutations of a multitude of genes, including the genes for p53 and retinoblastoma, can lead to tumor progression and metastasis. Most prostate cancers (95%) are adenocarcinomas.2
Etiology
Environmental risk factors include cigarette smoking, a diet high in fat or chromium, and obesity. Elevated levels of luteinizing hormone and of testosterone/dihydrotestosterone ratios are associated with mildly increased risk. Genetic changes associated with poor survival in prostate cancer include loss of one or both copies of the tumor suppressor gene PTEN, TMPRSS2-ERG chromosome fusion, P53 mutations, and overexpression of MYC. Prospective trials are needed to assess these markers more thoroughly before their implementation in clinical management. Currently, none of them is measured in routine practice.5
DIAGNOSIS
Asymptomatic Men
Digital rectal examination and PSA evaluation are the two components necessary for a modern screening program. The indications for screening are controversial. In 2011, a USPSTF draft statement recommended against PSA screening of all ages who do not have symptoms considered highly suspicious for prostate cancer.6 The American Cancer Society recommends that PSA evaluation and digital rectal examination (DRE) be offered annually, beginning at age 50 years to men who have at least a 10-year life expectancy, and that they should be offered as early as age 40 years to high-risk men. Information should be provided to patients regarding potential risks and benefits of intervention. Risks of screening include diagnosis and treatment of a clinically insignificant cancer, resulting in complications such as impotence or incontinence. A 2010 study concluded that in the 75- to 80-year age group discontinuation of PSA screening may be safe in African American men, with an initial PSA level of less than 6.0 ng per mL and in Caucasian men with an initial PSA of less than 3.0 ng per mL, because men in these groups are unlikely to develop high-risk prostate cancer.7
Symptomatic Men
Prostate cancer usually causes no symptoms in its early stages. When they occur, symptoms may include urinary frequency, urgency, hesitancy, or nocturia. If the cancer invades the neurovascular bundle, erectile dysfunction may result. Hematuria and hemospermia are relatively uncommon presenting symptoms. When metastatic, prostate cancer may cause bone pain or other symptoms due to distant lesions. When older men present with new symptoms of urinary obstruction or with symptoms that could be caused by bony metastases, the diagnosis of prostate cancer should be considered.8
Physical Examination
DRE is part of the diagnostic evaluation for prostate cancer for symptomatic men. On DRE, asymmetric areas of induration or frank nodules that are detected in the posterior and lateral aspects of the prostate gland are suggestive of prostate cancer. Tumors not detected by DRE include the 25% to 35% occurring in other parts of the gland and small, T1, cancers that are not palpable.8
Laboratory Studies
PSA is a protein made solely by prostate cells, so the antigen is highly specific for the prostate. However, it is not prostate cancer specific, and other prostate conditions, such as benign prostatic hyperplasia (BPH) or prostatitis, can affect PSA levels. The lack of specificity for prostate cancer has led to considerable controversy about the role of routine PSA testing. The controversy is compounded by the knowledge that not all cancers detected by routine screening require treatment. PSA levels generally increase with age and prostate size. The norm for a man less than age 50 years is <1 ng per mL, whereas it is >3 ng per mL for men over the age of 60 years. Some researchers advocate race-specific reference ranges.9
Monitoring
When PSA values are slightly elevated and DRE is normal, biopsy is sometimes deferred in favor of close monitoring of PSA values over time. In these cases, PSA may be initially repeated in a month or two and then again every 6 to 12 months. The change in PSA levels over time is referred to as PSA velocity. PSA exists in free and bound forms in the serum, with more bound PSA secreted by glands that have disrupted cellular architecture. As the proportion of free-to-total PSA drops below 20%, the likelihood of prostate cancer increases. Therefore, free PSA is often ordered to help decide whether to obtain a biopsy in borderline cases. Generally, PSA should be repeated before a biopsy is recommended since almost a third of patients will have a decrease to baseline levels if PSA is repeated a month or so later.9
Imaging
Transrectal ultrasound (TRUS) is not an accurate screening tool, but is used to guide needle biopsies that are done for diagnostic purposes. Pelvic and abdominal computed tomography (CT) and radionuclide bone scans are ordered when there is clinical suspicion of regional or distant metastases. Conventional endorectal magnetic resonance imaging (MRI) is helpful for localizing cancer within the prostate and seminal vesicles and for local staging. Dynamic, contrast-enhanced MRI and MR spectroscopic imaging are complementary in local staging, but their use is currently limited to a research setting.10
Surgical Diagnostic Procedures
Diagnosis of prostate cancer is typically made with the aid of a transrectal core needle biopsy. Biopsies focus on abnormal areas found on DRE and TRUS and also include multiple specimens covering all areas of the gland. Benign findings are often followed by repeat biopsy when the clinical diagnosis remains in doubt. The most common side effects of prostate biopsy are transient hemospermia and hematuria.10
Tumor Stage
The American Joint Committee on Cancer (AJCC) has developed a prostate cancer staging system based on the TNM (tumor, nodes, metastases) system. Stages 1 and 2 are defined, respectively, as nonpalpable and palpable tumors confined to the prostate. Stage 3 tumors extend through the prostate capsule and may involve the seminal vesicles but not the lymph nodes or other adjacent structures. Stage 4 tumors invade adjacent structures or have spread to regional lymph nodes or more distant sites.11
Gleason Score
Tumors may be well differentiated or poorly differentiated at any stage, and the level of tumor differentiation can be graded with a score of 1 to 5, with 5 being the most poorly differentiated. The Gleason score is the sum of grades for the two most prevalent patterns of differentiation seen on biopsy. A Gleason score of 4 or less is classified as a low-grade tumor. Scores of 5 to 7 are intermediate, and scores of 8 or above are high grade. The Gleason score adds prognostic information that can be used to guide treatment decisions, especially for Stage 1 and Stage 2 tumors.10
Clinical Staging
At present, approximately 90% of prostate cancers are localized at the time of diagnosis, and complete staging with bone scans, CT scans, and other imaging tests are usually reserved for patients who are clinically suspected to have a cancer that has spread beyond the prostate gland, such as in the case of a PSA >10 ng per mL or a high Gleason score. Seminal vesicle biopsy and pelvic lymph node dissection (PLND, which can now be done laparoscopically) are also sometimes used for more definitive clinical staging when needed to guide treatment.12
Differential Diagnosis
In asymptomatic men with a normal DRE, a mildly elevated PSA has a relatively low specificity and may be due to BPH, chronic prostatitis, or increased age. Local symptoms from prostate cancer can sometimes mimic BPH and include urinary frequency, hesitancy, nocturia, and weak stream. When these symptoms develop rapidly, they may be more indicative of cancer. When infection is suspected, an empiric trial of antibiotics with repeat PSA testing after treatment is sometimes tried before referring a patient for biopsy. Clinicians should consider the possibility of metastatic prostate cancer in older men presenting with new atypical back pain or other bone pain syndromes.13
TREATMENT
General Treatment Recommendations for Prostate Cancer
Selecting initial treatment requires assessing the risk of the disease spreading or progressing, which is based on evaluating life expectancy, comorbidities, biopsy grade (Gleason score), clinical stage, and PSA level.14
Treatment Recommendations for Clinically Localized Prostate Cancer
Very Low Risk of Recurrence
Patients with clinical stage T1c, Gleason score ≤6, PSA <10 ng per mL, fewer than three positive prostate cores, with a life expectancy <20 years, should be treated with active surveillance and observation.14
Low Risk of Recurrence
Patients with clinical stage T1–T2a, Gleason score of 2 to 6, PSA <10 ng per mL, with a life expectancy <10 years, should be treated with active surveillance.
Treatment of patients with a life expectancy ≥10 years includes active surveillance OR radical prostatectomy (RP) with or without PLND if predicted probability of lymph node metastases ≥2%. Patients with low-risk cancer are not candidates for pelvic lymph node irradiation or androgen deprivation therapy (ADT).14
Intermediate Risk of Recurrence
Patients with clinical stage T2b–T2c, Gleason score 7, and PSA 10 to 20 ng per mL, who have a life expectancy <10 years should be treated with active surveillance OR radiation therapy with daily image-guided radiotherapy (IGRT) with or without short-term ADT for 4 to 6 months with or without brachytherapy. Patients with a life expectancy ≥10 years should be treated with RP with PLND. Administering ADT before, during, and after radiation prolongs survival.14
High Risk of Recurrence
Patients with clinical stage T3a, Gleason score 8 to 10, and PSA >20 ng per mL should be treated with radiation therapy plus long-term ADT for 2 to 3 years or radiation therapy with daily IGRT plus brachytherapy.
Alternative treatment recommendations for localized prostate cancer include cryotherapy, high-intensity focused ultrasound, and particle beam therapy.14
Treatment Recommendations for Locally Advanced Prostate Cancer
Very High Risk
Clinical stage T3b–T4 treatment options include radiation therapy with IMRT plus long-term ADT for 2- to 3-year OR radiation therapy with IMRT with daily IGRT plus brachytherapy with or without short-term ADT.14
Metastatic Disease
Any T, N1 treatment includes ADT OR radiation therapy with IMRT with IGRT plus long-term ADT for 2 to 3 years. Any T, any N, M1 treatment should include only ADT.
Bisphosphonates are recommended for all men with hormone-refractory prostate cancer and bone metastases that have been shown to reduce pathologic bone fracture.14
Referrals
Treatment of prostate cancer often involves a variety of specialists, including a urologic oncologist and a radiation oncologist. Clear communication with the patient and treating physicians has the potential to help patients navigate complex diagnostic and treatment decisions.
Follow-Up
No randomized trials have yet defined the optimal surveillance strategy following treatment therapies for localized prostate cancer.
The standard follow-up for all patients is PSA testing and clinical evaluation. Current follow-up recommendations for patients who have undergone definitive therapy for localized disease are to monitor the serum PSA every 6 to 12 months for 5 years and then annually thereafter. Routine imaging procedures are not indicated in the absence of symptoms or a rising serum PSA.
The current recommendation for patients with metastatic prostate cancer is to monitor for disease progression and the side effects of long-term ADT. Patients should keep up with physician’s appointments and measurements of serum PSA level every 3 to 6 months.15
REFERENCES
1. Cornelis F, Rigou G, Le Bras Y, et al. Real-time contrast-enhanced transrectal US-guided prostate biopsy: diagnostic accuracy in men with previously negative biopsy results and positive MR imaging findings. Radiology 2013;269(1):159–166.
2. Jamal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106–130.
3. Siegel R, Naishadham D, Jemal A, Cancer statistics, 2013. CA Cancer J Clin 2013;63:11.
4. American Cancer Society. Cancer Facts and Figures 2010. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2010/index. Accessed November 20, 2013.
5. Markert EK, Mmizuno H, Vazquez A, et al. Molecular classification of prostate cancer using curated expression signatures. Proc Natl Acad Sci USA 2011:108(52):21276–21281.
6. Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155(11):762–771.
7. Tang P, Sun L, Robertson CN, et al. Prostate specific antigen-based risk-adapted discontinuation of prostate cancer screening in elderly African American and Caucasian American men. Urology 2010;76(5):1058–1062.
8. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:917–929.
9. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostate cancer. JAMA 1995;273:289–294.
10. Choi WW, Williams SB, Gu X, et al. Overuse of imaging for staging low risk prostate cancer. J Urol 2011;185(5):1645–1649.
11. Greene FL, Page DL, Fleming ID, et al, eds. AJCC cancer staging handbook. 6th ed. New York, NY: Springer; 2002:309–316.
12. Bostwick DG. Grading prostate cancer. Am J Clin Pathol 1994;102:S38–S56.
13. Smith DA, Catalona WJ. Rate of change in serum prostate specific antigen levels as a method for prostate cancer detection. J Urol 1994;152:1163–1167.
14. Ghavamian R. Prostate Cancer Treatment Protocols. http://emedicine.medscape.com/article/2007095-overview. Accessed November 20, 2013.
15. Penson DF, Vogelzang N, Lee WR, et al. Follow-up surveillance during and after treatment for prostate cancer. http://www.uptdate.com/contents/follow-up-surveillance-during-and-after-treatment-for-prostate-cancer. Accessed November 6, 2013.
|
General Principles
Chronic kidney disease (CKD) is defined as “abnormalities of kidney structure of function, present for >3 months, with implications for health.”
Criteria for CKD include either of the following for >3 months:
• Decreased glomerular filtration rate (GFR) <60 mL/min/1.73 m2
• Markers of kidney damage (one or more):
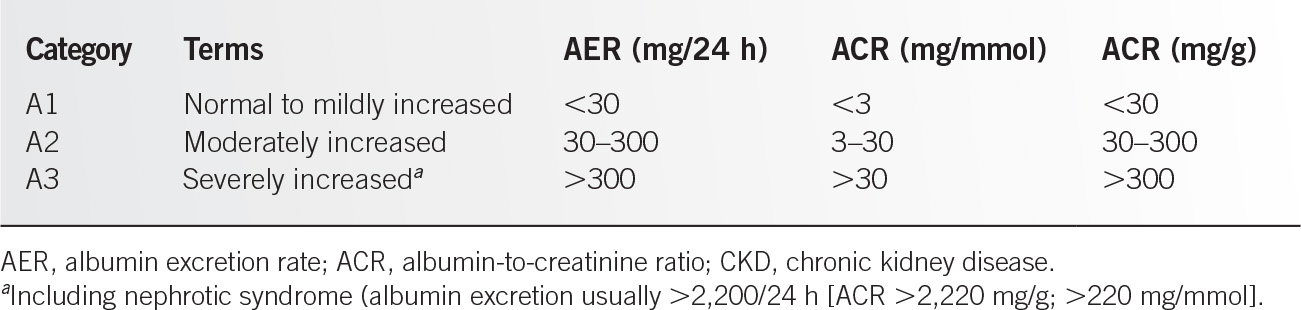
1. Albuminuria (albumin excretion rate [AER] ≥30 mg per 24 hours; albumin-to-creatinine ratio [ACR] ≥30 mg per g [≥3 mg per mmol])
2. Urine sediment abnormalities
3. Electrolyte and other abnormalities due to tubular disorders
4. Abnormalities detected by histology
5. Structural abnormalities detected by imaging
6. History of kidney transplant.1,2
Epidemiology
It is estimated that greater than 10% of adults (greater than 20 million people) have CKD of varying severity. Prevalence increases after the age of 50 and is most common in adults greater than age 70. It is present in one-third of adults with diabetes and one-fifth of adults with hypertension. Diabetic and hypertensive patients make up approximately 70% of new cases of end-stage renal disease (ESRD) in the United States. African Americans are about 3.5 times more likely to develop ESRD than whites, and Hispanics are about 1.5 times more likely to develop ESRD than non-Hispanics.3
Risk Factors
Clinical Factors
• Diabetes
• Hypertension
• Autoimmune disease
• Systemic infections
• Urinary tract infections
• Urinary stones
• Lower urinary tract obstruction
• Neoplasia
• Family history of CKD
• Recovery from acute kidney injury (AKI)
• Reduction in kidney mass
• Exposure to certain drugs
• Low birth weight
Sociodemographic Factors
• Older age
• U.S. ethnic minority status: African American, American Indian, Hispanic, Asian or Pacific Islander
• Exposure to certain chemical and environmental conditions (lead, cadmium, arsenic, mercury, uranium)
• Low income or education1,2,4
Classification
See Tables 12.7-1 and 12.7-2.1
GFR Categories in CKD1 |
GFR category | Terms | GFR (mL/min/1.73 m2) |
G1a | Normal or high | ≥90 |
G2a | Mildly decreased | 60–89 |
G3a G3b | Mildly to moderately decreased Moderately to severely decreased | 45–59 30–44 |
G4 | Severely decreased | 15–29 |
G5 | Kidney failure | <15 |
GFR, glomerular filtration rate.
aIn the absence of evidence of kidney damage, neither G1 nor G2 fulfills the criteria for CKD.
DIAGNOSIS
History
Many patients with CKD are asymptomatic and are found incidentally on routine laboratories or imaging studies performed for other reasons. Symptoms associated with CKD do not typically occur until the final stages (i.e., Stages 3 to 5) in the disease progression and more profoundly when kidney function deterioration is substantial enough to produce uremia (i.e., GFR less than 15 mL/min/1.73 m2). These symptoms could include generalized fatigue, symptoms associated with anemia, volume depletion or volume excess, and symptoms consistent with uremia, including generalized weakness, anorexia, nausea, vomiting, or mental status change. Bone pain is also common late in the course of CKD. Many of the earlier historical findings are related to the initiating events, underlying disease, or other risk factors listed above. History to determine cardiovascular disease risk and smoking status should also be explored.4–6
Physical Examination
Abnormal physical findings associated with CKD occur late in the process (i.e., Stage 5), except those related to underlying disease processes. Late findings include edema; hypertension; decreased urine output; pallor; easy bruising; cardiac dysrhythmias induced by hyperkalemia; asterixis, seizures, mental status change; and other neurologic manifestations of uremia. Physical evidence of hypovolemia, heart failure, or other causes of kidney hypoperfusion would suggest the potential of acute renal failure in the setting of CKD. Hypertension and presence of an abdominal bruit, findings consistent with peripheral vascular disease or carotid artery disease could suggest renal vascular disease. Acute muscle tenderness and swelling may indicate muscle injury. Abdominal, pelvic, and prostate evaluation may reveal the source of postrenal obstruction. Musculoskeletal findings such as arthritis or synovitis, fever, skin rash, or pulmonary lesions could suggest an autoimmune disease or vasculitis as an etiology for CKD.4,6
Laboratory and Imaging
Initial evaluation of CKD should include serum creatinine with GFR estimation, serum electrolytes, glucose, urine sediment, urine ACR, and renal ultrasound. A fasting lipid panel and EKG should be included to assess cardiovascular disease risk.
Creatinine secretion increases with advancing CKD, but may be normal in the earliest stages of CKD. An initial doubling of baseline serum creatinine levels may represent as much as a 50% loss of kidney function even when the doubling of the serum creatinine results in levels still within normal ranges. Measurement of serum creatinine levels alone to assess renal function does not account for differences caused by age, ethnicity, gender, weight, muscle mass, protein intake, or protein loss. GFR estimate equations that take these variables into account will more accurately determine renal function. Serum creatinine levels and GFR estimates performed serially over time can help determine whether the renal damage is a chronic or acute process, and can approximate the rate of kidney function decline in later stages of CKD. Blood urea nitrogen (BUN) increase is at 10:1 relationship with creatinine for that portion of BUN related to declining GFR (nonrenal causes of BUN elevation include gastrointestinal bleeding, high protein diets, and enhanced tissue destruction).
The preferred method for evaluating proteinuria is a urine ACR. Other tests (in decreasing order of preference) include urine protein-to-creatinine ratio, reagent strip urinalysis for total protein with automated reading, and reagent strip urinalysis for total protein with manual reading. Confirmatory testing (repeating ACR on an early morning sample or albumin or total protein excretion rate in a timed urine sample) is indicated if factors that can affect protein excretion without kidney damage are present. These factors include menstrual blood contamination, UTI, exercise, upright posture in orthostatic proteinuria, septicemia, intrinsic biological or genetic variability, degradation of albumin before analysis due to freezing, age (lower in children and elderly), race (lower in Caucasian than black individuals); muscle mass (lower in people with amputations, paraplegia, muscular dystrophy, or other causes of muscle atrophy); gender (lower in females); AKI; and samples with very high albumin concentrations (may be falsely reported as low or normal using some assays).3
Diminished kidney capacity to concentrate urine and maximally acidify urine, as well as conserve sodium in response to decreased effective circulating volume, is an indicator of advanced CKD. Urine sediment examination may reveal red blood cells and casts in certain glomerulopathies and white blood cells and casts in kidney infections.
Renal ultrasound will reveal the presence of small echogenic kidneys in late CKD stages with normal or large kidneys in earlier stages and large kidneys caused by hyperfiltration in early disease. Ultrasound can also reveal the presence of urinary tract obstruction and abnormalities of kidney parenchyma (e.g., cysts). Plain imaging may reveal evidence of nephrocalcinosis. Computed tomography (CT) scan of the abdomen can provide better resolution of kidney structural changes. Contrasted imaging studies should be avoided. Captopril renal scans may be used in detecting the presence of renal artery stenosis at early stages of CKD.
Other tests to identify rare causes of CKD can include serum protein electrophoresis for plasma cell dyscrasias, antinuclear antibody (ANA), and dsDNA antibody for systemic lupus erythematosis and consumption of serum complement for certain autoimmune glomerulopathies and antiglomerular basement membrane for Goodpasture syndrome.
To identify progression of CKD, assessment of GFR and albuminuria should be obtained at least annually, but should occur more frequently in individuals at higher risk of progression and/or when measurement will impact therapeutic decisions. When GFR falls below 60 (CKD 3 to 5), the patient should also be evaluated for complications of CKD including anemia, malnutrition, mineral and bone disorders, neuropathy, and overall decreased level of functioning and well-being.1,2,4,6
Differential Diagnosis
See Table 12.7-3.7,8
TREATMENT
Management of chronic renal disease begins with the recognition of individuals who are at risk for this problem before the presence of markers that indicate early renal dysfunction. Interventions to control hypertension and diabetes and avoidance of renal-toxic agents should be perceived as kidney-protective strategies. Interventions aimed at slowing the progression of CKD included control of hypertension, hyperglycemia, and proteinuria; reducing protein intake; as well as implementing strategies for cardiovascular risk reduction to include appropriate exercise, weight loss, reduce sodium intake, control of dyslipidemias, and smoking cessation in both diabetic and nondiabetic nephropathy. Although the cardiovascular risk reduction activities have not been shown to be associated with slowing the progression of CKD, their impact on cardiovascular health is essential since patients with CKD have accelerated risk for cardiovascular disease events.1,2,5–7
Hypertension
Chronic and uncontrolled hypertension can accelerate the loss of kidney function. Strict blood pressure (BP) control is recommended for both diabetic and nondiabetic nephropathy since evidence shows that these interventions slow progression of CKD. According to JNC 8 guideline, BP target for patients 18 years and older with CKD is less than 140/90. Treatment should be initiated if BP is greater than 140/90. There is no evidence to recommend a specific BP goal for people aged 70 years and older with GFR <60. Initial or add on antihypertensive treatment should include angiotensin-converting enzyme inhibitors or angiotensin II blocking agents to improve kidney outcomes regardless of race or diabetes status, but they should not be used together. Usually all agents can be employed to control hypertension in patients with CKD. Thiazide diuretics and, in advancing disease (GFR <30 to 40), loop diuretics may be required to lower BP, reduce sodium retention, and provide additional kaluresis in response to the associated hyperkalemia.9–11
Acute kidney injury Alport syndrome Amyloidal kidney Chronic glomerulonephritis Chronic pyelonephritis Congenital abnormality of metabolism Diabetic nephropathy Drug induced nephropathy Goodpasture syndrome Gouty kidney Malignant hypertension Multiple myeloma Nephropathy of pregnancy/toxemia | Nephrosclerosis Obstructive urinary tract disease Polycystic kidney disease Rapidly progressive glomerulonephritis Renal artery stenosis Renal hypoplasia Renal/urinary tract tuberculosis Renal/urinary tract calculus Renal/urinary tract tumor Systemic lupus erythematosus nephritis Wegener granulomatosis Other unclassifiable nephritis |
Glycemic Control
Tight glycemic control to hemoglobin A1C HbA1c to about 7.0% has been shown to slow the progression of microvascular complications and CKD. Target hemoglobin A1C should be extended above 7.0% in individuals with comorbidities, limited life expectancy, and risk of hypoglycemia.1,2,12,13
Microalbuminuria and Macroalbuminuria
Testing for microalbuminuria in diabetic patients should be performed annually as it allows detection of diabetic nephropathy at an earlier stage before a decline in GFR or an elevation in BUN and creatinine occurs. The use of ACE inhibitors and angiotensin II receptor blockers (ARBs) when microalbuminuria or macroalbuminuria is detected has been shown to slow the progression of diabetic and nondiabetic kidney disease independent of the presence of systemic hypertension. One must carefully monitor these agents to avoid hyperkalemia especially in advancing stages of CKD. ACE inhibitors and ARBs are not indicated for primary prevention of kidney disease diabetic patients without hypertension or albuminuria.1,2,13,14
Hyperlipidemia
Although the effect of the control of hypercholesterolemia on the progression of CKD is not known, the increase association of coronary artery disease (CAD) with patients who have CKD is a compelling reason to lower low-density lipoprotein (LDL) levels to decrease atherogenesis.
In patients age ≥50 years with CKD and GFR >60, Statin is recommended. In patients age ≥50 years and GFR <60 (not posttransplant), treat with a Statin or Statin/ezetimibe combination. In patients age 18 to 49 with CKD without dialysis or transplant, treat with Statin if one or more of the following risk factors are present: known CAD, diabetes, prior ischemic stroke, and estimated 10-year cardiovascular risk >10%. For patients on dialysis, do not initiate treatment for hypercholesterolemia, but it may be continued if patients are already receiving treatment. Statin treatment is recommended in adult renal transplant recipients. There is no evidence to suggest LDL target is beneficial. Therefore, the DKIGO work group suggests to not measure follow-up LDL unless the result would alter management.1,2,13,15
Dietary Restriction
Patients with CKD should receive a formal dietary assessment and advice tailored to the severity of CKD. Reasonable protein restriction that avoids protein malnutrition individualized to each patient should be employed although there is no evidence to suggest any impact on the progression of chronic CKD. The restriction of dietary protein to 0.6 to 0.8 g/kg/day in patients with GFR <50; and 0.3 to 0.5 g/kg/day in patients with GFR <20 who are not on dialysis is recommended. Caloric intake should be between 23 and 35 kcal/kg/day in patients with CKD. In patients with CKD Stages 3 to 5, sodium and potassium should be restricted to <2 g per day, phosphorus should be restricted to 800 to 1000 mg per day, and calcium to 2000 mg per day. Vitamin D should be replaced to maintain adequate serum levels.1,2,16
Mineral and Bone Disorders (Renal Osteodystrophy and Extraskeletal/Vascular Calcification)
Phosphate retention occurs early in CKD and worsens as GFR decreases, which causes a fall in serum calcium levels. Hypocalcemia is exacerbated by decreased intestinal calcium absorption due to decrease renal synthesis of 1,25(OH)D. Hypocalcemia and hyperphosphatemia indirectly stimulate parathyroid hormone (PTH) release, which leads to bone disease, vascular calcification, cardiovascular disease, and increased mortality.
In patients with GFR less than 45 mL/min/1.73 m2, testing should include serum calcium, phosphate, PTH, alkaline phosphatase, and vitamin D levels. Although fracture rates and fracture-related mortality are elevated in CKD, routine bone density testing is not recommended in patients with GFR less than 45 mL/min/1.73 m2 as the information may be inaccurate and misleading. Monitoring phosphate levels, restricting phosphate intake, and using phosphate binders to maintain normal phosphorus levels will ameliorate some of the negative impact of PTH elevation and calcium lowering, and decrease the development of renal osteodystrophy and extracellular/vascular calcification. Vitamin D replacement is only recommended with documented deficiency, not solely to suppress PTH in non-dialysis patients. In vitamin D–deficient patients, replacement increases Bone Mineral Density (BMD) and muscle strength, reduces fracture risk and falls, and reduces PTH. Bisphosphonate therapy is recommended for treatment of osteoporosis and/or high fracture risk in CKD patients with GFR greater than 60 mL/min/1.73 m2 and GFR 30 to 60 mL/min/1.73 m2 with a normal PTH. Dose modification may be necessary.1,2,5
Hyperkalemia
When GFR falls below 20 mL per minute (i.e., Stages 4 and 5), hyperkalemia occurs because of diminished kidney potassium excretory capacity, especially with acute potassium loads. Potassium intake should be restricted as noted above. If hyperkalemia occurs, the source of excess potassium intake, decreased potassium excretion, or cellular extrusion of potassium should be eliminated. Further treatment of persistent and severe hyperkalemia is directed at antagonizing myocardial effects by using calcium gluconate, shifting potassium intracellularly with glucose and insulin or with sodium bicarbonate if metabolic acidosis is severe, removal of potassium-sparing diuretics, and use of ion-exchange resins, for example, sodium polystyrene sulfate and, in emergency settings, dialysis.1,2,5
Metabolic Acidosis and Sodium and Water Hemostasis
The acidosis, hypervolemia, and hyponatremia of CKD, which usually starts in Stage 4, require the use of sodium bicarbonate when pH is less than 7.3 and serum bicarbonate concentrate (HCO3−) is less than 22 mEq per L. Sodium chloride restriction should be implemented based on the clinical setting as noted above. Water restriction may be required in patients who develop hyponatremia when they consume free water at rates greater than the kidney water clearance rate.1,2
Hyperuricemia
Hyperuricemia (uric acid level greater than 7 mg per dL) is common in CKD patients, and there is a growing body of evidence to suggest an association with hyperuricemia in CKD and adverse cardiovascular outcomes. However, there is insufficient evidence to currently support or refute the use of uric acid–lowering agents in individuals with CKD and either symptomatic or asymptomatic hyperuricemia for the specific goal of delaying progression of CKD.1,2
Anemia
Anemia of CKD usually starts at Stage 3 and is due to declining erythropoietin production causing a decrease in red blood cell (RBC) production. Partial correction of the anemia of kidney disease improves the quality of life in predialysis and dialysis patients although there is no evidence it changes the progression of CKD.
• Evaluate for other causes with complete blood count, absolute reticulocyte count, serum ferritin, transferrin saturation, B12, and folate. Search for source of blood loss.
• Oral or IV iron is recommended if serum ferritin <100 ng per mL and transferrin saturation <20%.
• Erythropoiesis-stimulating agents (ESAs) are recommended with Hgb <10 g per dl and when transfusion is anticipated due to rapid hemoglobin decline. Goal Hgb is between 9 and 11.5 g per dL with ESA treatment.
• Transfusion if iron and ESA are ineffective; risk if ESA outweighs benefit; acute hemorrhage; unstable CAD; rapid pre-op Hgb correction is required. No specific Hgb threshold is recommended. Treat if the patient is symptomatic.
Monitoring
• CKD with anemia not on ESA: every 3 months with CKD Stage 3 to 5 non-dialysis, or Stage 5 on peritoneal dialysis. Monthly for CKD Stage 5 on hemodialysis.
• CKD with anemia on ESA: monthly.1,2,17,18
Medication Management and Patient Safety
Prescribers should always take GFR into account when dosing medications for CKD patients. Consultation with a pharmacist is advantageous in this setting, and to provide counseling to patients regarding their prescribed and over-the-counter medications. Temporary discontinuation of potentially nephrotoxic and renally excreted drugs (including, but not limited to ACE-Is, ARBs, aldosterone inhibitors, direct renin inhibitors, diuretics, NSAIDs, metformin, lithium, and digoxin) is recommended in patients with GFR less than 60 mL/min/1.73 m2 who have serious illness that increases the risk of AKI. Herbal agents should be avoided. Metformin should be discontinued once the GFR falls below 30 mL/min/1.73 m2, but can be used with caution with GFR 30 to 44 mL/min/1.73 m2. If the GFR is 45 mL/min/1.73 m2 or higher, metformin should be continued. For all CKD patients on potentially nephrotoxic agents, GFR, electrolyte, and drug levels should be monitored regularly.1,2
Immunizations
All patients with CKD should receive a yearly influenza vaccine. Pneumococcal and hepatitis B vaccines are recommended in patients with a GFR less than 30 mL/min/1.73 m2, and those at high risk. Immunologic response to hepatitis B should be confirmed with serologic testing. Consideration of live vaccines should include an appreciation of the patients’ immune status and should follow CDC guidelines.1,2
Referrals
Patients with CKD should be referred to specialist for further evaluation in any of the following case.
• CKD Stages 4 and 5
• Unsure etiology
• Heavy proteinuria with ACR ≥70 mg per mmol unless it is due to diabetes or already treated
• Proteinuria with ACR ≥30 mg per mmol along with hematuria
• Active urine sediment
• Rapidly declining estimate of GFR (eGFR) (more than 5 mL/min/1.73 m2 in 1 year or more than 10 mL/min/1.73 m2 within 5 years).
• Patient with rare or genetic cause of disease.
• Resistant hypertension or suspected renal artery stenosis
• Difficult management issues: anemia, secondary hyperparathyroidism metabolic bone disease, and electrolyte disturbance.1,2,14
Kidney Replacement Therapy
Consultation with a kidney team should occur early in the predialysis period to promote transition of patients to kidney replacement therapy in a nonurgent setting. This early contact with the kidney team ensures that patients have an appropriate understanding of the types of kidney replacement therapies available to them. The kidney team can also affect the quality of life of the predialysis kidney patient by providing specific patient education and assessments concerning medical treatment of CKD as well as treatment of associated problems. Current kidney replacement therapies include hemodialysis, intermittent peritoneal dialysis, continuous ambulatory peritoneal dialysis, and kidney transplantation. The choice of kidney replacement therapy should be individualized based on the availability of a donor kidney, the patient’s desire for independence, previous abdominal surgeries, underlying medical conditions, and patient’s age. Initiation of dialysis (which usually occurs with GFR 5 to 10 mL/min/1.73 m2) is indicated for one or more of the following: signs or symptoms due to kidney failure (uremia, serositis, acid–base or electrolyte abnormalities, pruritis); inability to control volume status or BP; progressive deterioration in nutritional status refractory to dietary interventions; or encephalopathy.1,2
REFERENCES
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;84(Suppl 3):1–150.
2. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2013;63(5):713–735.
3. Centers for Disease Control and Prevention (CDC). National Chronic Kidney Disease Fact Sheet: General Information and National Estimates on Chronic Kidney Disease in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. http://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf. Updated September 18, 2012. Accessed August 22, 2014.
4. Baumgarten M, Gehr T. Chronic kidney disease: detection and evaluation. Am Fam Physician 2011;84(10):1138–1148.
5. Rosenberg M. Overview of the Management of Chronic Kidney Disease in Adults. UpToDate. http://www.uptodate.com/contents/overview-of-the-management-of-chronic-kidney-disease-in-adults. Updated May 16, 2014. Accessed September 1, 2014.
6. Fatehi P, Hsu C. Diagnostic Approach to the Patient with Acute Kidney Injury (Acute Renal Failure) or Chronic Kidney Disease. UpToDate. http://www.uptodate.com/contents/diagnostic-approach-to-the-patient-with-acute-kidney-injury-acute-renal-failure-or-chronic-kidney-disease. Updated October 27, 2014. Accessed September 1, 2014.
7. Arora P. Chronic Kidney Disease. Medscape. http://emedicine.medscape.com/article/238798-overview. Updated September 9, 2014. Accessed September 15, 2014.
8. Uchida S. Differential Diagnosis of Chronic Kidney Disease (CKD): by primary diseases. Japan Med Assn J 2011;54(1):22–26.
9. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012;60(5):850–886.
10. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507–520. doi:10.1001/jama.2013.284427.
11. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012;2(5):337–414.
12. Taler SJ, Agarwal R, Bakris GL, et al. National Kidney Foundation KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis 2013;62(2):201–213. © 2013 by the National Kidney Foundation, Inc.
13. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012;60(5):850–886.
14. Standards of medical care in diabetes. VI. Prevention and management of diabetes complications. Diabetes Care 2013;36(Suppl 1):S28–S39.
15. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl 2013;3(3):259–305.
16. American Dietetic Association. Chronic kidney disease evidence-based nutrition practice guideline. Chicago (IL): American Dietetic Association; 2010 Jun. Accessed at http://www.guideline.gov/content.aspx?id=23924 on October 27, 2014.
17. U.S. Food and Drug Administration. FDA drug safety communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Accessed September 1, 2011.
18. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012;2(4):279–335.
|
GENERAL PRINCIPLES
• Definition. Urolithiasis refers to calculi (stones) in any part of the urinary tract.1,2 Kidney stones, nephrolithiasis, or renal calculi are terms commonly used in place of urolithiasis.
• Anatomy. At the time of diagnosis, stones may be found in any part of the urinary tract, from the minor calyces in the kidney to the urethra.2
• Classification. Stones may be of any composition from calcium (calcium oxalate, the most common, and calcium phosphate), uric acid, struvite (magnesium ammonium phosphate), or cystine. It is possible one patient may produce more than one type or combination of stone.3
• Epidemiology. Kidney stones are relatively common, affecting approximately 1% of the population, with men being affected 2:1 over women.1,2 Most renal calculi are found in the third through the fifth decade of life.2 For patients affected, reoccurrence rates approach 50%.1 Stones composed of calcium make up nearly 80% of all stones.3 Calcium stones are more common in men than in women. Struvite stones, which represent 10% to 15% of all urinary calculi, are more common in women, as they are often the result of urinary tract infections (UTIs) from urease-producing bacteria.1,2 Uric acid stones represent 5% to 8% of all urinary calculi and are more common in men as well; this may be because gout is more common in men and half of all patients with uric acid stones have gout.1,2 Cystine stones are rare, representing 1% of all calculi, and occur in men and women with equal prevalence.2 All stones are radiopaque except uric acid stones, which are radiolucent.2
• Pathophysiology. Kidney stones are thought to form secondary to any process that alters the kidney’s delicate balance of prolithogenic factors and factors inhibiting the formation of stones, thus allowing a stone to precipitate out of solution in the urine. Stone-forming factors include the following: increased dietary oxalate, decreased fluid intake, increased dietary protein (specifically from animals), increased dietary sodium, and high-dose vitamin C.1 In addition to these easily modifiable risk factors, there are other difficult-to-modify risk factors such as hypercalciuria, hyperoxaluria, primary hyperparathyroidism, hypocitraturia, and hyperuricosuria.1,3 Major risk factors to developing a calcium oxalate stone in addition to the preceding include prior history of a stone and family history of stones. Uric acid stones are thought to arise from saturation of urate crystals in the urine. Fifty percent of people with uric acid stones also have gout.2 Struvite stones are thought to form as the result of urease-producing bacteria, especially the Proteus species. These stones can become quite large and have the potential to become staghorn calculi, that is, calculi that fill and obstruct the entire renal pelvis.
• Mechanism of injury. Stones in the renal pelvis can be an incidental finding in asymptomatic patients.4 Stones become painful when they become lodged in the urinary tract and the body attempts to move them along. Contraction of the ureter against the stone produces a severe, colicky pain. Often, there is some bleeding associated with this, found as gross or microscopic hematuria.
DIAGNOSIS
• Clinical presentation. Stones present with acute onset of severe colicky pain. The pain is often unilateral and its location depends on the location of the stone in the urinary tract. The pain is often referred to the cutaneous areas T11–L2 that supply the ureter and is variably described as flank pain that commonly radiates to the testicle or labia on the ipsilateral side.3,5 The pain moves as the stone moves, and a person with previous stones may be able to tell if the stone is about to pass. Patients often find it difficult to find a comfortable position and may readjust frequently, trying to find stated position. Hematuria is present in up to 90% of stones, but its absence does not rule out a stone.3 Nausea and vomiting often accompany the pain. If the stone is located in the distal ureter, dysuria, frequency, and urgency may also be present.2
• Physical examination. Patients have a lack of physical examination findings other than the pain. Tachycardia, diaphoresis, and hypertension as a result of the pain may be present. Fever is absent unless a concurrent urinary tract infection is also present. Flank pain is present, but not made worse by costovertebral angle percussion.
• Differential diagnosis. An acute onset of flank or abdominal pain has a broad differential diagnosis, including multiple organ systems. Renal cell carcinoma may bleed and clot off a ureter, thus producing renal colic.3 Other illnesses that may present with vague abdominal pain but are not classically colicky in nature, such as acute appendicitis, diverticulitis, or abdominal aneurysm. Other colicky types of pain include biliary colic or an ectopic pregnancy. Last, renal colic may be faked by patients for secondary gain in those seeking narcotics.3
• Laboratory studies. Urinalysis looking for blood and the presence of infection are routinely obtained. No other abnormalities should be expected in a case of simple, first-time episode of nephrolithiasis in the acute setting. However, a metabolic panel is commonly obtained to check renal function and electrolytes like calcium.2
• Imaging. The current gold standard for diagnosis of a kidney stone is helical computed tomography (CT) scan without contrast. The specificity of a helical CT with 3- to 5-mm cuts is 98% and sensitivity 95% in one study, and specificity is consistently close to 100% in various studies.3 CT imaging also allows the physician to see any obstruction caused by the stone and will aid in the differential diagnosis of the pain if a stone is not present. Kidney–ureter–bladder (KUB) radiography will not demonstrate radiolucent stones, such as uric acid stones.3 Intravenous pyelogram (IVP) is the next best choice; however, it is neither as sensitive nor as specific, and it is often a slower test. Ultrasound may also be used to detect urinary tract obstruction and radiolucent stones. Ultrasound can be used safely during pregnancy. The combination of ultrasound with KUB may provide comparable results to CT alone.3
• Monitoring. It should be noted that the time for passage of each stone is variable and there is no set time limit.4 Stones may pass in a little as a few hours, or it may take weeks. In a patient with a distal stone less than 5 mm with pain well controlled, the physician may well continue to observe for a month or more.
• Pathologic findings. After a stone has been diagnosed, patients should be asked to strain their urine for collection of the stone.4 The stone should then be sent for stone analysis. The type of stone will guide treatment and strategies for prevention of reoccurrence.
TREATMENT
• Medications. Narcotics are often required to control the pain in the acute setting. Nonsteroidal anti-inflammatory drugs (NSAIDs) are also effective. Both indomethacin and ketorolac have been used in the acute setting alone and in combination with narcotics. Anti-emetics and IV fluids are also frequently used. If a stone is expected to pass, the patient may be sent home with either opiate and/or NSAIDs for pain control. To aid in the passage of the stone, α-blockers and calcium-channel blockers are thought to help decrease tone throughout the urinary tract and allow stones to pass more easily.3,5 Meta-analysis shows that α-blockers are superior to calcium-channel blockers and are the preferred agents.6 If infection or obstruction is present, an antibiotic like a fluoroquinolone should be started.
• Surgery. Urology consult is warranted if there is acute kidney injury, obstruction, urosepsis, stones larger than 5 mm, a patient fails outpatient therapy, or if the pain is uncontrollable.3 Size is the single best predictor if a stone will pass, and a stone of 4 mm or less will most likely pass on its own.3 In one study, stones 5 to 7 mm have a passage rate of 60%.3
• Nonoperative. Nonoperative treatments include shock wave lithotripsy and active surveillance.2,4
• Operative. Surgical treatment modalities include ureteroscopic lithotripsy, laparoscopy, and percutaneous nephrolithotomy.4 Ureter stents may also be placed to facilitate stone passage.
• Counseling. Prevention of reoccurrence is based largely on the type of stone.1 For nearly all stone types, increased daily water intake of approximately 2 to 3 L is the single best recommendation to prevent recurrence. Patients may also be counseled to modify their diet based on the type of stone found. Medications may be suggested to patients based on their metabolic profile and the type of stone, as discussed below.
• Follow-up. For patients with recurrent stones, a 24-hour urine should be collected preferably 1 to 2 months after acute even and tested for urine volume and pH, and excretion of calcium, uric acid, citrate, oxalate, creatinine, and sodium.4 Serum calcium and parathyroid hormone are also helpful. Thiazide diuretic may reduce the amount of calcium present in the urine. Hypocitraturia may be treated with potassium citrate.2,7 Hyperuricosuria may benefit from a low-purine diet and prevent the occurrence of calcium oxalate stones.2 For patients with uric acid stones, allopurinol should be given.2 For patients with struvite stones, antimicrobial therapy and surveillance may be beneficial.
SPECIAL CONSIDERATIONS
• Pregnancy. Kidney stones can occur during pregnancy. If the pregnancy is early on, an ectopic pregnancy must be ruled out. During pregnancy, there is an increase in calcium excretion in the urine relative to urine volume, so a simple increase in glomerular filtration rate cannot account for the increase.8 Despite this, nephrolithiasis is a rare event. When reviewing imaging studies, it must be kept in mind that the normal pregnant patient will have a dilation of the ureters secondary to progesterone-mediated smooth muscle relaxation. Stones will often pass secondary to this relative dilation.8 Opiates can be used for pain during pregnancy while NSAIDs are not recommended.
• Children. Management of stones in children is very similar to adults. Narcotics, NSAIDs, α-blockers, anti-emetics, and IV fluids are all used in children. Guidelines for stone passage and referral to urologist are also similar. Radiation exposure should be considered when ordering imaging. There has been some evidence of kidney stones in children treated long term with ceftriaxone.3
ACKNOWLEDGMENT
The author would like to acknowledge Michael A. Green for his work on the previous version of this chapter.
REFERENCES
1. Mount DB, Loscalzo J. Nephrolithiasis. In Dan L, Anthony F, Dennis K, Stephen H, Jameson J, Joseph L Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2013:998–1000.
2. Wesson J. Nephrolithiasis. In: Dario MT. Kochar’s Clinical Medicine for Students. 5th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2009:535–538.
3. Curhan GC, Aronson MD, Preminger GM. Diagnosis and Acute Management of Suspected Nephrolithiasis in Adults. UpToDate. http://www.uptodate.com/contents/diagnosis-and-acute-management-of-suspected-nephrolithiasis-in-adults? Updated June 1, 2013. Accessed January 2, 2014.
4. Preminger GM, Curhan GC. The First Kidney Stone and Asymptomatic Nephrolithiasis in Adults. UpToDate. http://www.uptodate.com/contents/the-first-kidney-stone-and-asymptomatic-nephrolithiasis-in-adults? Updated 19 June 2013. Accessed January 2, 2014.
5. Lendvay TS, Smith J, Stapleton FB. Acute Management of Nephrolithiasis in Children. UpToDate. http://www.uptodate.com/contents/acute-management-of-nephrolithiasis-in-children? Updated June 18, 2013. Accessed January 2, 2014.
6. Preminger GM, Tiselius HG, Assimos DG, et al; EAU/AUA Nephrolithiasis Guideline Panel. 2007 guideline for the management of ureteral calculi. J Urol 2007;178:2418–2434.
7. Curhan GC. Prevention of Recurrent Calcium Stones. www.uptodate.com. Accessed January 2, 2014.
8. Rose B. Nephrolithiasis During Pregnancy. www.uptodate.com. Accessed January 2, 2014.
|
BACKGROUND
Urinary incontinence (UI), an involuntary, unintended leakage of urine, is a common condition with prevalence reported at 16.2% to 81.9% affecting 20 million adults in the United States, with prevalence increasing with age. Although not a lethal condition, it is associated with great medical costs, increased risk of skin breakdown, falls, low self-esteem, social isolation, and depression. The average cost per individual in the United States with Stress UI is $5,624 per treatment.1–5
GENERAL PRINCIPLES
The cause of UI can typically be determined by the primary care provider through a thorough history and targeted physical examination. Diagnostic studies may be indicated for more complicated cases of UI, but imaging is not routinely recommended in the initial therapy. The detailed history, alone, is often telling enough to formulate a plan of care. However, the physician must remember to ask about this common condition as embarrassment associated with UI may interfere with patients volunteering this information.4–6 Postvoid residuals (PVRs) are generally not useful in an initial evaluation. Specific factors including urinary retention, recent pelvic surgery, and neurologic disease warrant PVR testing. In addition, because of cost and difficulty of testing, urodynamic testing is not recommended for initial testing. Other imaging is generally not indicated. In addition to a UI for all cases, laboratory testing is not necessary unless a specific pathology is thought to be contributing.7
CLASSIFICATIONS
The classification of UI is based on the associated pathophysiologic abnormality, and patients may have features of any mixture of these types.
• Transient UI has abrupt onset and resolves when the underlying condition is treated. The causes of transient UI can be remembered by the mnemonic DIAPERS (delirium, infection, atrophic urethritis, pharmaceutical, excessive urinary output, restricted mobility, and stool impaction).4
• Chronic UI can be divided into four types: stress UI, urge UI, overflow UI, and functional UI.
• Stress UI occurs when pressure within the bladder exceeds bladder sphincter pressures. These commonly occur during times of increased intra-abdominal pressure such as with sneezing, coughing, or changing sitting/standing positions.8 Patients will describe loss of small volumes of urine and PVR volumes are usually normal (<50 mL). Stress UI is the most common type and affects approximately 35% of those with UI. This common urinary complaint is essentially a sphincter disorder caused by pelvic floor muscular relaxation or sphincter/bladder outlet incompetence from prior instrumentation (i.e., obstetrical repair) or prostate surgery.4 The history alone is usually diagnostic and sufficient. A bladder stress test and PVR can confirm the diagnosis.7
![]() Behavioral therapy: Along with weight loss, the initial treatment strategy for men and women with stress UI is the pelvic floor strengthening, or “Kegel” exercises. These exercises can produce positive results in up to 38% of stress UI. There are a number of exercise regimens and in general comprehensive, clinic based and longer training produces better results.2,3
Behavioral therapy: Along with weight loss, the initial treatment strategy for men and women with stress UI is the pelvic floor strengthening, or “Kegel” exercises. These exercises can produce positive results in up to 38% of stress UI. There are a number of exercise regimens and in general comprehensive, clinic based and longer training produces better results.2,3
![]() Pharmacologic therapy: There are no FDA-approved medications for stress UI.2 Although topical estrogen is widely used to increase urethral thickness and sensitive α-adrenergic receptors in the urethral sphincter, a meta-analysis of studies of estrogen effect on stress UI found no improvement in urine loss. This lack of evidence on efficacy and concerns about estrogen supplementation posed by the Women’s Health Initiative make estrogen a poor choice for the treatment of stress UI.9 Pseudoephedrine, phenylephrine, and duloxetine all have limited evidence and adverse effects in the treatment of stress UI.2,8
Pharmacologic therapy: There are no FDA-approved medications for stress UI.2 Although topical estrogen is widely used to increase urethral thickness and sensitive α-adrenergic receptors in the urethral sphincter, a meta-analysis of studies of estrogen effect on stress UI found no improvement in urine loss. This lack of evidence on efficacy and concerns about estrogen supplementation posed by the Women’s Health Initiative make estrogen a poor choice for the treatment of stress UI.9 Pseudoephedrine, phenylephrine, and duloxetine all have limited evidence and adverse effects in the treatment of stress UI.2,8
![]() Other modalities: Stimulation of the pelvic floor with noninvasive electrical and magnetic stimulation along with a myriad of minimally invasive procedures has been met with varying success. Pessaries have few contraindications and remain a viable option for treatment.2
Other modalities: Stimulation of the pelvic floor with noninvasive electrical and magnetic stimulation along with a myriad of minimally invasive procedures has been met with varying success. Pessaries have few contraindications and remain a viable option for treatment.2
![]() Surgical options: After conservative therapy has failed, surgical therapy including slings and urethroplexy as directed by a urogynecologist are further options for treatment of stress UI.2,3
Surgical options: After conservative therapy has failed, surgical therapy including slings and urethroplexy as directed by a urogynecologist are further options for treatment of stress UI.2,3
• Urge incontinence is the leakage of often large amounts of urine and the inability to delay voiding after the sensation of bladder fullness is detected. Like stress UI, urge UI typically involves normal PVR volumes. This type of UI is often associated with neurologic disorders, such as dementia or cerebrovascular disease. Most patients with UI, however, do not have a neurologic disease, and this type is the most common form of UI experienced by older adults. This disorder is due to detrusor hyperactivity.4,8
![]() Behavioral therapy: Scheduled toileting at increasing intervals can aid in increasing compliance in the bladder over time. Bladder training with learning to control detrusor urge by learning to sit still and allow the bladder contraction to pass, typically less than 60 seconds, and only then move to the restroom to void along with Kegel exercises all remain first-line therapy.2
Behavioral therapy: Scheduled toileting at increasing intervals can aid in increasing compliance in the bladder over time. Bladder training with learning to control detrusor urge by learning to sit still and allow the bladder contraction to pass, typically less than 60 seconds, and only then move to the restroom to void along with Kegel exercises all remain first-line therapy.2
![]() Pharmacologic therapy: Best used with behavioral therapy, medications focus on decreasing detrusor over activity. A number of anticholinergic drugs are available. Pharmacologic therapy is limited by anticholinergic side effects, such as dry mouth, delirium, and constipation. Mirabegron is a β-adrenergic agonist not to be used with uncontrolled hypertension. Botox to the detrusor can relieve symptoms for up to 6 months.2,8,10
Pharmacologic therapy: Best used with behavioral therapy, medications focus on decreasing detrusor over activity. A number of anticholinergic drugs are available. Pharmacologic therapy is limited by anticholinergic side effects, such as dry mouth, delirium, and constipation. Mirabegron is a β-adrenergic agonist not to be used with uncontrolled hypertension. Botox to the detrusor can relieve symptoms for up to 6 months.2,8,10
![]() Electrical therapy: Office based-tibial nerve stimulators have good results in three quarters of patients. Surgically implanted devices, most commonly a sacral nerve stimulator inserted into the tissue of the lower back or buttocks may improve UI by stimulating the S3 sacral nerve and decrease detrusor muscle contractility.2
Electrical therapy: Office based-tibial nerve stimulators have good results in three quarters of patients. Surgically implanted devices, most commonly a sacral nerve stimulator inserted into the tissue of the lower back or buttocks may improve UI by stimulating the S3 sacral nerve and decrease detrusor muscle contractility.2
• Overflow UI is the frequent or continuous leakage from mechanical forces over a distended/full bladder or from other effects of urinary retention on bladder or sphincter function. PVRs are usually high (>100 mL). This type of UI is often a consequence of bladder outlet obstruction from prostatic enlargement. Another cause of overflow UI is bladder hypoactivity, referred to as “neurogenic bladder.” This is most commonly encountered in patients with spinal cord injury, long-standing diabetes mellitus, or vitamin B12 deficiency. Anticholinergic medications may induce a hypoactive bladder and medication review is crucial. Therapy is directed by the etiology.4,5
![]() Bladder outlet obstruction: The target of medicinal therapy is relaxation of the internal urethral sphincter with α-adrenergic-blocking agents and bladder decompression as described below. Available α-blockers include terazosin (Hytrin), doxazosin (Cardura), and tamsulosin (Flomax). Orthostatic hypotension is the typical limiting side effect of the α-blockers and may be less problematic with the more selective tamsulosin than the other agents. Transurethral resection of the prostate may be warranted for patients who do not respond well to pharmacologic treatment or who are limited by orthostasis.5,8
Bladder outlet obstruction: The target of medicinal therapy is relaxation of the internal urethral sphincter with α-adrenergic-blocking agents and bladder decompression as described below. Available α-blockers include terazosin (Hytrin), doxazosin (Cardura), and tamsulosin (Flomax). Orthostatic hypotension is the typical limiting side effect of the α-blockers and may be less problematic with the more selective tamsulosin than the other agents. Transurethral resection of the prostate may be warranted for patients who do not respond well to pharmacologic treatment or who are limited by orthostasis.5,8
![]() Medication side effect: Removal of the offending agent and decompression with an indwelling Foley catheter for 7 to 14 days may allow the bladder to resume some contractile activity.
Medication side effect: Removal of the offending agent and decompression with an indwelling Foley catheter for 7 to 14 days may allow the bladder to resume some contractile activity.
![]() Neurogenic bladder: This type of overflow UI responds poorly to pharmacologic therapies. Patients may successfully manage their voids by being taught to do intermittent straight catheterization several times a day or placement of a chronic indwelling catheter may be considered.5
Neurogenic bladder: This type of overflow UI responds poorly to pharmacologic therapies. Patients may successfully manage their voids by being taught to do intermittent straight catheterization several times a day or placement of a chronic indwelling catheter may be considered.5
• Functional UI occurs in patients with normal bladder function from some extrinsic cause. Detrusor and sphincter function are intact, but the patient is either unable to recognize the urge to void or physically unable to get to the toilet on time. Debility, dementia, delirium, and cerebrovascular disease are common causes of functional UI.4
![]() There is no available pharmacotherapy for functional UI. Treatment focuses on scheduled toileting and the use of incontinence supplies. Chronic indwelling catheterization is not suggested but may be warranted when perineal or sacral wounds are present.4
There is no available pharmacotherapy for functional UI. Treatment focuses on scheduled toileting and the use of incontinence supplies. Chronic indwelling catheterization is not suggested but may be warranted when perineal or sacral wounds are present.4
REFERRAL
In addition to the above indications, referral should be considered with uncertain diagnosis, suspected cancer, neurologic disease, pelvic pain or mass, hematuria, previous surgery, and cases that do not respond to initial therapy.7
ACKNOWLEDGMENT
This author would like to thank Mary McDonald and Sarah Parrott, the authors of the previous version of this chapter, for their contributions.
REFERENCES
1. Sensoy N, Dogan N, Ozek B, et al. Urinary incontinence in women: prevalence rates and impact on quality of life. Pak J Med Sci 2013;29(3):818–822.
2. Hersh L, Salzman B. Clinical management of urinary incontinence in women. Am Fam Physician 2013;87(9):634–640.
3. Carpenter D, Visovsky C. Stress urinary incontinence: a review of treatment options. AORN J 2010;91(4):471–478.
4. Weiss BD. Diagnostic evaluation of urinary incontinence in geriatric patients. Am Fam Physician 1998;57(11):2675–2684.
5. Moore KN, Gray M. Urinary incontinence in men: current status and future directions. Nurs Res 2004;53(6):S36–S41.
6. Artibani W, Cerruto M. The role of imaging in urinary incontinence. BJU Int 2005;95:699–703.
7. DuBeau CE. Approach to women with urinary incontinence. In: Falk S, ed. UpToDate. 2013. http://www.uptodate.com. Accessed March 12, 2014.
8. Moreleand RB, Brioni JD, Sullivan JP. Emerging pharmacologic approaches for the treatment of lower urinary tract disorders. J Pharmacol Exp Ther 2004;308(3):797–804.
9. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 2002;288:321–333.
10. Jayarajan J, Radomski S. Pharmacotherapy of overactive bladder in adults: a review of efficacy, tolerability, and quality of life. Res Rep Urol 2014;6:1–16.
|
GENERAL PRINCIPLES
The term acute renal failure has now been replaced by acute kidney injury (AKI) to highlight the fact that even small decreases in renal function that do not result in significant organ damage are associated with increased morbidity and mortality.1–5
Definition
AKI is a sudden loss of kidney function resulting in increase in serum creatinine or decrease in urine output, leading to accumulation of waste products (usually <48 hours). The Kidney Disease/Improving Global Outcomes (KDIGO) AKI Workgroup defined the following criteria:
• Increase in SCr by ≥0.3 mg per dL (≥26.5 μmol per L) within 48 hours; or
• Increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or
• Urine volume <0.5 mL/kg/h for 6 hours.5
Epidemiology
There are limited data on overall epidemiology of AKI due to underreporting, regional disparities, and inconsistency of definition. Prevalence of AKI is on the rise worldwide. In the United States, prevalence ranges from 1% in the community up to approximately 7% of all hospital admissions. Five percent to 20% of critically ill patients experience an episode of AKI during the course of their illness, 4% to 9% of which require renal replacement therapy (RRT). Age plays a significant role in the epidemiology of AKI around the world. Although it is mostly seen in the elderly population in developed countries, younger adults and children are more affected in developing countries.3,6–8
Classification
See Table 12.10-1.
DIAGNOSIS
History
Obtaining a comprehensive history based on risk factors, noted in Table 12.10-2, is essential in the clinical assessment of the patient. Particular attention should be paid to assessing volume status, exposure to nephrotoxic substances, systemic illness, and underlying chronic conditions that could put the patient at risk for AKI. The clinical presentation varies with etiology and severity of renal injury, as well as any associated diseases. Patients with mild to moderate AKI are usually asymptomatic and are incidentally found on routine laboratory testing. Patients with severe cases may be symptomatic and present with listlessness, confusion, fatigue, anorexia, nausea, vomiting, weight gain, or edema. Patients may present with oliguria (urine output less than 400 mL per day), anuria (urine output less than 100 mL per day), or normal volumes of urine (nonoliguric AKI). Anuria suggests AKI due to one of the three causes: urinary tract obstruction, a severe type of acute tubular necrosis (ATN) called cortical necrosis, or blood vessel blockage by clot or other obstruction.
Staging of Acute Kidney Injury |
Stage | Serum creatinine | Urine output |
1 | 1.5–1.9 times baseline OR ≥ 0.3 mg/dL (≥26.5 μmol/L) increase | < 0.5 mL/kg/h for 6–12 h |
2 | 2.0–2.9 times baseline | <0.5 mL/kg/h for ≥12 h |
3 | 3.0 times baseline OR Increase in serum creat to ≥4.0 mg/dL (≥353.6 μmol/L) OR Requires renal replacement therapy OR Decrease in eGFR to <35 mL/min/1.73 m2 in pts <18 yrs | 0.3 mL/kg/h for ≥24 h OR anuria for ≥12 h |
Creat, creatinine; eGFR, estimated glomerular filtration rate.
Adapted from International Society of Nephrology. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl – J Int Soc Nephrol 2012;2:89–115.
Exposures and Susceptibilities for Nonspecific AKI |
Exposures | Susceptibles |
Sepsis Critical illness Circulatory shock Burns Trauma Cardiac surgery (especially cardiopulmonary bypass) Major noncardiac surgery Nephrotoxic drugs Radiocontrast agents Poisonous plants and animals | Dehydration or volume depletion Advanced age Female gender Black race Chronic kidney disease Chronic diseases (heart, lung, liver)
Diabetes Cancer Anemia Human immunodeficiency virus |
Adapted from International Society of Nephrology. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl – J Int Soc Nephrol 2012;2:89–115.
Assessment of prerenal AKI should include awareness of any recent volume loss (e.g., history of vomiting, diarrhea, diuretic overuse, hemorrhages, or burns), or any underlying cardiac or liver disease.
In intrinsic renal AKI, certain findings may suggest the cause and portion of the kidney affected. Patients currently taking any nephrotoxic medications (over the counter, illicit, or herbal) may suggest ATN. History of hypotension, trauma, or myalgia can suggest rhabdomyolysis, also pointing to ATN, as well as recent radiographic studies that required contrast agents. A history of systemic diseases as mentioned in Table 12.10-3, may suggest a glomerular component. Indications of interstitial causes include medication use, rashes, arthralgia, fevers, and infectious illness. Nephrotic syndrome, trauma, flank pain, anticoagulation, vessel catheterization, or vascular surgery in the history should lead one to consider a vascular cause.
Postrenal findings include urinary urgency or hesitancy or polyuria. Any history of gross hematuria, history of stones, any cancers of urinary and reproductive systems, and any previous urological or gynecologic surgeries may suggest a postrenal cause of AKI.2,9–11
Physical Examination
For physical findings, see Table 12.10-3.
Laboratory and Imaging
Initial laboratory evaluation should include serum electrolytes, blood urea nitrogen (BUN) and creatinine level, fractional excretion of sodium (FENa), urinalysis, and complete blood count. If the patient is on a Na-wasting diuretic, a fractional excretion of urea (FEUrea) should be ordered instead. Other laboratory indices helpful in a selected group of patients include BUN/serum creatinine ratio, rate of rise in serum creatinine concentration, urine osmolality, and urine volume.
Increased levels of BUN and creatinine are hallmarks of renal failure. Serum creatinine levels should be compared to previous values to determine duration and acuity of disease. AKI by definition is a rise in creatinine within 48 hours. In the outpatient setting, however, it may be difficult to be certain when the rise occurred. High serum creatinine level in a patient with previously normal levels suggests an acute process, whereas a rise over weeks to months represents subacute or chronic. As a general rule, if serum creatinine increases more than 1.5 mg/dL/day, rhabdomyolysis must be ruled out. Creatinine elevation is a late marker for renal dysfunction. Once creatinine is elevated, severe reduction in glomerular filtration rate has already occurred.
A ratio of BUN to creatinine can exceed 20:1 in conditions that favor enhanced reabsorption of urea, such as volume contraction (prerenal AKI). BUN can also be elevated with gastrointestinal and mucosal bleeding, steroid treatment, or protein loading.
Physical Examination Findings and Common Associated Diseases |
System | Examination findings | Common disease complications |
Skin | Livedo reticularis, digital ischemia, butterfly rash, palpable purpura Maculopapular rash Track marks (IV drug abuse) | Systemic vasculitis Allergic interstitial nephritis Endocarditis |
Eyes | Keratitis, iritis, uveitis, dry conjunctivae Jaundice Band keratopathy Signs of diabetes mellitus Signs of hypertension Atheroemboli | Autoimmune vasculitis Liver disease Multiple myeloma Retinopathy |
Cardiovascular system | Irregular rhythms (atrial fibrillation) Murmurs Pericardial friction rub Increased jugulovenous distention, rales, S3 heart sound | Thromboemboli Endocarditis Uremic pericarditis Heart failure |
Abdominal | Pulsatile mass or bruit Abdominal or costovertebral angle tenderness Pelvic, rectal masses; prostatic hypertrophy; distended bladder Limb ischemia, edema | Atheroemboli Nephrolithiasis, papillary necrosis, renal artery thrombosis, renal vein thrombosis Urinary obstruction Rhabdomyolysis |
Pulmonary | Rales Hemoptysis | Goodpasture syndrome, Wegener granulomatosis Wegener granulomatosis |
Modified from Workeneh BT, Agraharkar M, Gupta R. Acute Kidney Injury. Medscape. http://emedicine.medscape.com/article/243492-overview. Accessed November 25, 2013.
The measurement of FENa is helpful in distinguishing prerenal from intrinsic causes of AKI, especially in the setting of oliguria. A value of less than 1% indicates a prerenal cause of AKI. A value greater than 2% indicates an intrinsic renal cause. For patients currently on diuretic therapy, FENa is less reliable in prerenal states due to natriuresis caused by the diuretic. For these patients, fractional excretion of urea (FEUrea) may help, with values less than 35% indicating a prerenal cause.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree