Renal and Acid-Base Physiology
BODY FLUIDS
Explain the 60-40-20 rule.
Total body water (TBW), in liters, is 60% of body weight in kilograms, intracellular fluid (ICF) is 40% of body weight, and extracellular fluid (ECF) is 20% of body weight
What is the distribution of ECF in the human body?
ECF is one-third of TBW.
Plasma is one-fourth of ECF or one-twelfth of TBW.
Interstitial fluid is three-fourths of ECF or one-fourth of TBW.
What are the major cations of the ICF and ECF?
ICF: K+ and Mg2+
ECF: Na+
What are the major anions of the ICF and ECF?
ICF: protein and organophosphates
ECF: Cl– and HCO3–
What substance is used to measure the following major fluid compartments?
TBW
Tritiated H2O or D2O
ECF
Sulfate, inulin, or mannitol
Plasma
Radioiodinated serum albumin (RISA), Evans blue
Interstitial fluid (IF)
Indirectly: IF = ECF – plasma
ICF
Indirectly: ICF = TBW – ECF
What is the method for using the above listed substances to calculate these volumes?
The dilution method. A known amount of substance is administered to your patient and then given time to equilibrate in the volume of interest. Now if we collect a sample and again determine its concentration, we can calculate that volume.
C1 × V1 = C2 × V2
C1 = concentration of injected substance
V1 = volume of injected substance
C2 = concentration of measured sample
V2 = value to be calculated
RENAL BLOOD FLOW AND FILTRATION
What percentage of the cardiac output goes to the kidneys?
20%; the highest blood flow of any organ when calculated per gram tissue.
Describe the blood flow through the glomerulus:
Blood enters via the renal arteries and is shuttled through large arteries to the afferent arterioles. It then flows through the glomerular tuft and out into an efferent arteriole (not a venule).
Once blood passes through the efferent arteriole where does it go?
This efferent flow provides blood to the peritubular capillary beds, with some going to the vasa recta. Very little of the total renal blood flow (RBF) moves through the vasa recta.
What is autoregulation of RBF?
Process by which renal vasculature changes resistance to keep the glomerular blood flow and filtration pressures consistent.
Describe the hypothesized mechanisms by which autoregulation is achieved:
Myogenic mechanism: stretch receptors in renal arterioles detect rising pressures and cause the contraction of the afferent arterioles, to increase resistance, and maintain constant blood flow.
Tubuloglomerular feedback: the macula densa senses [Na+] changes and uses that as a measure of filtrate flow rate. It directs the afferent arterioles to change its resistance thereby changing flow.
What inflammatory cytokine is important in dilating the afferent arteriole?
Prostaglandins. These are used in the signaling pathway for vessel dilation. This is one of the reasons why the use of nonsteroidal anti-inflammatory drugs is dangerous in people with kidney disease who rely on higher filtration pressures to maintain GFR.
What hormone is important in constricting the efferent arteriole?
Angiotensin II. The use of ACE or ARB class antihypertensive drugs can be dangerous in patients with poor renal function, as it can reduce glomerular filtration pressures.
What are the components of the glomerular filtration barrier?
Fenestrated capillary endothelium
Fused basement membrane lined with heparan sulfate
Epithelial layer consisting of podocyte foot processes
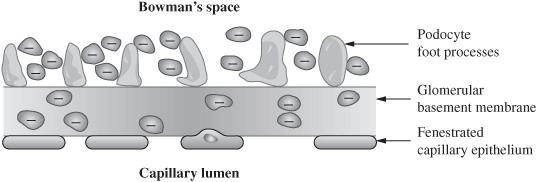
Figure 5.1 Anatomy of the renal filtration system.
What is the purpose of the fenestrated endothelium?
This is a very coarse filter and really only impedes RBC from moving out of the vessel
What does the basement membrane do?
It offers a “size barrier” to plasma proteins restricting all but the smallest proteins to the vascular space
What aspect of the filtration barrier prevents the smallest proteins from entering Bowman space?
Anionic glycoproteins (heparan sulfate) that line the basement membrane repel the negatively charged proteins (“charge barrier”)
What is “clearance”?
In physiology, it is the volume of plasma that is cleared (cleaned, if you will) of a substance per unit time.
What is the equation used to measure clearance (C) of the kidney?
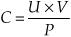
C= clearance
U = urine concentration of substance x
V= urine flow rate
P = plasma concentration of substance x
In words, what is the glomerular filtration rate (GFR)?
GFR is the volume of plasma per unit time that moves from the vascular space to Bowman space.
What can be used clinically to approximate GFR?
Creatinine (Cr) clearance
What makes creatinine clearance so well-suited to use in the clinical setting?
It is endogenously produced as a product of muscle turnover at a consistent rate for a given muscle mass, and it is filtered readily at the glomerulus and only minimally secreted or reabsorbed in the nephron
What is meant by the terms “filtered,” “secreted,” and “reabsorbed”? (we will return to this ad nauseum below)
Filtered: the quantity of solute that enters the tubular fluid (TF) at the Bowman space
Secreted: the quantity that is put into the TF as it moves through the nephron
Reabsorbed: the quantity that is removed from the TF and put back into the plasma
What substance is used to measure renal plasma flow (RPF)? Why?
Para-aminohippuric acid (PAH), it is filtered and secreted by renal tubules (so it gives us a measure of all blood moving through the kidney, not just the filtered fraction). Note that this is a research tool, primarily
How is RPF calculated?
RPF is measured as the clearance of PAH
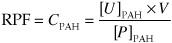
CPAH = clearance of PAH
[U]PAH = urine concentration of PAH
V= urine flow rate
[P]PAH = plasma concentration of PAH
How do you measure renal blood flow (RBF)?
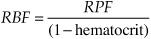
What is the filtration fraction (FF)?
The fraction of renal plasma flow that moves into Bowman space
How is FF calculated?
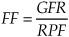
What is the normal value for FF?
Under physiologic conditions, one-fifth of RPF is filtered at the glomerulus, or about twenty percent.
What happens to the protein concentration in the peritubular capillaries with increasing FF?
Increases; as filtrate is removed in the glomerulus the remaining blood products become more concentrated
What substance is used to definitively measure the glomerular filtration rate (GFR)? Why?
Inulin: it is filtered but not reabsorbed or secreted by the renal tubules, also primarily a research tool
What equation is used to directly measure GFR?
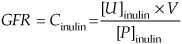
Using the clearance equation with inulin
Cinulin = clearance of inulin
[U]inulin = urine concentration of inulin
V = urine flow rate
[P]inulin = plasma concentration of inulin
How does falling GFR effect blood urea nitrogen (BUN) and plasma Cr?
Causes them to increase, since both are filtered by the glomerulus
Does GFR remain constant with aging?
No, it decreases with age
What happens to plasma Cr with age?
It remains relatively constant despite the decrease in GFR with age due to the decrease in muscle mass seen with aging. Less muscle means less turnover.
What is the Starling equation for GFR?
GFR = Kf[(PGC − PBS) − (πGC – πBS)]
Describe each of the following terms from the Starling equation:
Kf
Filtration coefficient across the glomerular barrier.
pGC
Hydrostatic pressure in the glomerular capillary. This is essentially the blood pressure of the glomerular capillaries and is determined by the resistance of the afferent and efferent arteriole.
pBS
Hydrostatic pressure in Bowman space, under normal physiologic conditions, this value should be very low.
πGC
Oncotic pressure in the glomerular capillary. It increases along the length of the glomerular capillary as filtrate is removed from the capillary.
πBS
Oncotic pressure in Bowman space, since protein is kept within the capillary, this value should be zero.
What can cause an increase in PGC? What is the effect on GFR?
Dilation of the afferent arteriole or constriction of the efferent arteriole. This elevated hydrostatic pressure will increase GFR.
What can cause an increase in PBS? What is the effect on GFR?
Any obstruction of the tubules or of the lower urinary tract. This increased pressure decreases GFR.
In a patient with cirrhosis, who has lost some hepatic synthetic capacity (fewer serum proteins), how is the πGC changed?
This will reduce the oncotic pressure, and increase the GFR
If the urine concentration of inulin is 0.25 mg/mL, the urine flow rate is 25 mL/min and the plasma concentration of the drug is 0.12 mg/mL, what is the renal clearance?
Using the equation for clearance
U = 0.25 mg/mL
V = 25 mL/min
P = 0.12 mg/mL
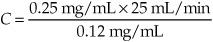
C = 52 mL/min
RENAL SECRETION, REABSORPTION, AND EXCRETION
Identify the portions of the nephron numbered in the diagram below.
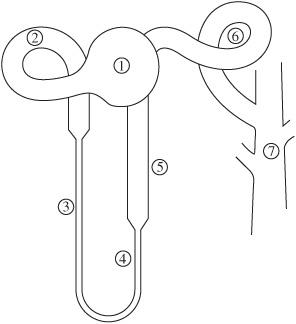
Figure 5.2 Nephron structure.
- Bowman space
- Proximal tubule
- Descending limb of the loop of Henle
- Thin ascending limb of the loop of Henle
- Thick ascending limb of the loop of Henle
- Distal convoluted tubule
- Collecting duct
How is the elimination rate of a given substance calculated?
Ey = (FLy + Sy)− Ry
Ey = Elimination rate of substance y
FLy = Filtration rate of substance y
Sy = Secretion rate of substance y
Ry = Reabsorption rate of substance y
How is the rate at which a molecule is filtered across the glomerular capillary calculated?
FLy = GFR × Py
FLy = Filtered load of substance y
Py = Substance plasma concentration
How is the rate of a molecule’s excretion in urine calculated?
Ey = Uy × V
Ey = Excreting rate of substance y
V = Urine flow rate
Uy = Concentration of substance in urine
How do you determine if a substance is ultimately secreted or absorbed?
Take the difference between rate excreted and rate filtered (excreted-filtered):
If > 0 → secretion
If < 0 → absorption
If = 0 → neither secreted nor absorbed
Will secreted substances have higher or lower clearance rates than the filtration rate?
Higher
Will reabsorbed substances have higher or lower clearance rates than the filtration rate?
Lower
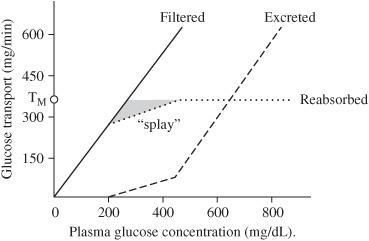
If we plot filtration and excretion rates across many plasma concentrations, what do we generate?
Renal titration curves. These can be plotted for any renally handled substance.
As an example, let us consider glucose:
Is glucose normally secreted or reabsorbed?
Reabsorbed
How?
There are very efficient Na+-glucose cotransporters found in the proximal tubules which mediate glucose uptake
At what concentration of glucose does the Na+-glucose carriers in the proximal tubules start to become saturated? What happens to any glucose above this level?
Approximately 250 mg/dL; once this level is exceeded some glucose begins to spill into the urine as the cotransporters become saturated. At 350 mg/dL glucose uptake is saturated and all increases in filtered glucose will be excreted
What is it called when the kidney begins to excrete a substance that should be conserved (250 mg/dL for glucose)?
Renal threshold, above this point, glucose transport in some of the nephrons is saturated, so glucose begins to appear in urine.
What is the term for the glucose concentration where the maximum rate of solute transport is achieved?
Transport maximum (Tm). Note that different sources will give you different values for threshold and Tm.
Figure 5.3 Glucose titration curve.
What is splay?
The region on a titration curve between the renal threshold and Tm. In this region some nephrons have maximized their transport capacity for glucose, others, because of larger population of transporters, have not.
What is the splay for glucose?
Between 250 to 350 mg/dL
As another example, let us look at the renal handling of PAH:
What happens to the filtered load of PAH as the plasma load of PAH increases?
Increases in direct proportion to the plasma load
Is PAH secreted or reabsorbed?
Secreted
Where does this occur?
Proximal tubules
What is the mechanism by which PAH is secreted?
Specialized carriers in the proximal tubules mediate transcellular transfer of PAH into the tubular fluid (TF).
What happens to secretion of PAH when Tm is reached?
It plateau’s and any further increase in plasma PAH will not change the secretion rate
How is excretion calculated for PAH (and other secreted substances)?
We modify the elimination formula to calculate PAH excretion. Since PAH is filtered and secreted, but not reabsorbed, the formula simplifies to:
EPAH = (FLPAH + SPAH)
When using PAH to measure RBF, what important limitation must be appreciated?
RPF can only be measured at plasma concentrations < Tm

Figure 5.4 Titration curve for PAH.
PAH, then, has a very high elimination rate that approaches 100%. What are some examples of substances with very low elimination rates?
Na+
Glucose
Amino acids
HCO3–
Cl−
What does the ratio of [x]TF to [x]P represent (i.e. the TF/P ratio)?
It compares the concentration of a substance in TF with that of plasma
What is the utility of this value?
It allows us to interpret renal handling of a given substance; that is, does the body want to get rid of it, hold on to it, etc.
What does each of the following ratios represent?
TF/P = 1
Either there is no reabsorption of the substance or reabsorption of the substance is equal to that of water
TF/P <
Reabsorption of a substance is greater than water, thus concentration of substance x in TF is less than plasma
TF/P >
Reabsorption of a substance is less than water, thus concentration of substance x in TF is greater than plasma
What substance can be used as a marker for water reabsorption along the nephron and why?
Inulin.
Because inulin is freely filtered, but it is neither secreted nor reabsorbed, so its concentration in TF changes only as a consequence of water reabsorption
Na+ and K+ Handling
In renal water management, what is the primary electrolyte that we pay attention to clinically?
Sodium (Na+); “water always follows salt”
Give the percentage of Na+ reabsorption along the following parts of the nephron:
Proximal convoluted tubule (PCT)
67%
Thin descending limb
0%
Thin ascending limb
0%
Thick ascending limb
25%
Distal convoluted tubule (DCT)
5%
Collecting ducts (CDs)
3%
What percentage of all of the filtered Na+ is excreted in a normal nephron?
1% to 2%, depending on an individual’s total salt intake
In the early proximal tubule, what major substances are preferentially reabsorbed with Na+ by cotransport?
Glucose
Amino acids
Phosphate Lactate
Which one of these coupled solutes is most important for Na+ recovery?
No one solute; they all play an important role in the sodium handling of the proximal tubule.
How is HCO3− reabsorbed in the proximal tubule?
Via carbonic anhydrase, which converts it to CO2 that can move transcellularly. This is the solute that gets the most attention in the proximal tubule, likely because it’s the one that we can most potently influence pharmacologically.
At physiologic values, what percentage of glucose and amino acids are reabsorbed in the proximal tubule?
100%
Is Na+ reabsorbed at a constant or variable rate in the proximal tubule?
Essentially constant; 67% of all filtered Na+ is reabsorbed
What is the name of the mechanism by which this is accomplished?
Glomerulotubular balance
What forces influence glomerulotubular balance?
The same Starling forces that balance fluid filtration at the glomerulus also influence the peritubular capillary blood vessels. It works to balance glomerular filtration.
What is the effect of the following on reabsorption?
(Think: Starling forces)
Increased GFR
Increased FF
Increased: in both cases, the removal of more plasma volume at the glomerulus results in hemoconcentration and a resultant increase in protein concentration
ECF volume contraction
Increased: by increase in peritubular protein concentration and decrease in peritubular pressure
ECF volume expansion
Decreased: by decrease in peritubular protein concentration and increase in peritubular pressure
Describe the mechanism by which Na+-H+ exchange occurs in the proximal tubule:
Na+ is reabsorbed by coupled exchange with H+. This process is directly linked to HCO3− reabsorption (see below).
What brush border enzyme is responsible for the Na+/HCO3 reabsorption mechanism?
Carbonic anhydrase
Generally speaking, how is Na+ reabsorbed in the late proximal tubule?
Na+ is reabsorbed with Cl–, although the various segments use different transporters to accomplish this.
What happens in the descending limb and the thin ascending limb of the loop of Henle?
There is no net reabsorption of water here; the movement of water and electrolytes helps to generate an osmotic gradient in the medulla of the kidney (more later)
What transporter is responsible for reabsorption of Na+ in the thick ascending limb of the loop of Henle?
Na+ -K+ -2Cl− cotransporter in the luminal membrane
What diuretics are responsible for inhibition of this transporter?
Loop diuretics: furosemide, ethacrynic acid, and bumetanide
Is the thick ascending limb of the loop of Henle permeable to water?
No, therefore, NaCl is reabsorbed without water thereby diluting the tubular fluid.
What happens to the osmolarity of the TF and [Na+] in the thick ascending limb compared to plasma?
Decreases, which is why the segment is called the diluting segment
What change can be seen in transluminal electrical potential in this segment?
The luminal fluid gains a slight net positive charge; the Na-K-2Cl cotransport removes two negative and two positive charges making transport electroneutral.
However, a luminal K+ channel (the romK channel) allows for backleak of positive charge across the luminal membrane leading to a net positive tubular fluid (more on this in other e-lytes)
Why is this luminal positive charge important?
It is important in establishing a driving force for paracellular cationic efflux from the tubular fluid (Ca++, Mg++, etc.)
What transporter is responsible for Na+ reabsorption in the early distal tubule?
Na+ -Cl− cotransporter
What diuretics work on the transporter in this segment?
Thiazide diuretics
Is the early distal tubule permeable to water?
No
What happens to the osmolarity of the solution in the tubular lumen in this section?
It becomes further diluted
Name the cell types responsible for electrolyte transport in the late distal tubule and CDs.
Principal cells and α-intercalated cells
In principal cells, what major electrolytes are secreted and which are reabsorbed?
Secreted: K+
Reabsorbed: Na+ and H2O
What is the other important hormone that influences principal cells?
ADH (vasopressin)
How does ADH work?
It directs the insertion of water channels (aquaporins) into principal cell luminal membranes
What effect does aldosterone have on electrolyte reabsorption or secretion?
Increases Na+ reabsorption and K+ secretion
2%, but this is the important 2% that fine tunes sodium balance
What percentage of overall Na+ reabsorption is affected by aldosterone?
What diuretics work on principal cells?
K+ -sparing diuretics (spironolactone, triamterene, amiloride), which block sodium channels and sodium channel insertion preventing sodium reabsorption and, indirectly, decreasing K+ secretion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree