, Mohammed Al-Rubaie2, Stuart Walker3 and Sam Salek4
(1)
Regulatory Affairs Consultant Executive Directive, Kuwait Advancement for Conference and Exhibition Management, Kuwait, Kuwait
(2)
Ministry of Health, Directorate General of Pharmaceutical Affairs and Drug Control, Muscat, Oman
(3)
Founder of Centre for Innovation in Regulatory Science, London, UK
(4)
Department of Pharmacy School Life & Medical Sciences, University of Hertfordshire, Hatfield, UK
Introduction
Long regulatory approval timelines for new medicines delay their access to the market that may have the potential to improve patients’ health status and of alleviating suffering. Variation in the availability of medicines in different countries has been studied since the early 1970s (Rawson 2000), and some marked differences have been identified. Regulatory approval time is a key metric that is used to evaluate the performance of regulatory agencies. A study was previously carried out to evaluate the milestones and timelines involved in the regulatory review processes for different authorities (Hirako et al. 2007). A specific study of the Gulf regulatory authorities (Hashan 2005) highlighted important aspects of the drug approval procedures in each of the seven member states. However, the study provided limited information about the approval timelines and the duration of the milestones and stages involved in the review process because the authorities did not have electronic tracking systems to monitor such activities. This made it difficult, if not impossible, to allow cohorts of pharmaceutical products to be monitored retrospectively.
Currently, within the GCC states, there are two registration systems, the national and the centralised procedure. In order to benchmark and make true comparisons between the different authorities, it is essential to identify first the common processes that occur during registration. However, there are no major differences in the legislation, regulations or requirements for marketing pharmaceutical products in the GCC states (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, UAE and Yemen). All the national regulatory departments in the GCC states have regulations that govern and regulate most aspects of the pharmaceutical services by effective law. Apart from the registration process of medicinal products, some countries also have price control in their remit.
It is recognised that individual authorities have different experiences and knowledge that could be of value to other member states through comparison of various systems and sharing of best practices to the benefit of all. This study was therefore conducted to explore the timelines of the approval processes in the GCC states. However, it is important to have a basic understanding of the regulatory review process in these countries in order to place the approval time in an appropriate context.
The five GCC authorities responsible for the regulation of pharmaceutical products in the Gulf region participated in this study, namely, Bahrain, Kuwait, Oman, Qatar and Saudi Arabia. Yemen was excluded from this study because they could not provide data due to the unstable political situation in the country during the period of this study and because of the marked difference in their socioeconomic status compared to other Gulf states. The United Arab Emirates was also excluded from this study, as they were unable to furnish the full data due to changes in the drug regulatory organisation that took place during the time this study was conducted. Additionally, they were also unable to extract some of the data, as it was manually stored, making retrieval of the required information very difficult.
A face-to-face meeting with all the Directors and General Directors of Regulatory Affairs in the Ministry of Health in the GCC states took place during the GCC-DR meetings in 2011 and 2012. They provided the data on approval timelines as an Excel spreadsheet including the registration data for products approved in 2008, 2009 and 2010, based on the milestones in each authority. The data consisting of all products from Gulf, Arab non-Gulf, Asian and international manufacturers, approved for human use in 2008, 2009 and 2010 in the GCC states, were included in this study. The products reviewed included both New Active Substances (NASs) and Existing Active Substances (EASs) and only those with a complete data set. The regulatory approval times were calculated as the time from submission of the product dossier to the regulatory authority until the final approval. The data sets included the total number of registered products between 2008 and 2010 and regulatory approval time in GCC states between 2008 and 2010 for different groups of pharmaceutical products (GCC, non-GCC Arabs, international and Asian).
Three major areas were examined, for the period 2008–2010, namely, (1) trends in submission and registration of new pharmaceutical products, (2) changes in the approval timelines and (3) approval times for Gulf, Arab non-Gulf, Asian and international pharmaceutical products in the GCC states. The number of approved products in any regulatory authority is affected by many factors and varies from year to year. Factors that influence the number of products approved include the number of dossiers submitted to the authority and the number of reviewers in the review section (Hashan 2005).
Total Number of Products Approved in Each Gulf State
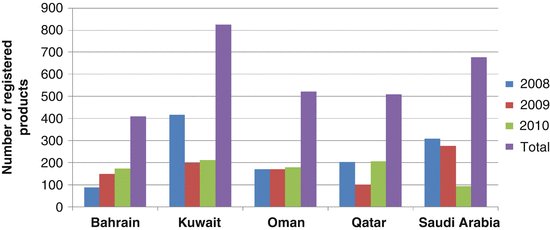
In general, the countries reviewed a similar number of products, but there was an increase in the number of approved products in 2010 in most GCC states compared to 2008 and 2009 with the exception of Saudi Arabia (Table 3.1). This may reflect an increased interest by the industry in the Gulf market or the authorities in the region were able to cope with an increased workload, translating into an increased number of products approved. The GCC regulatory authorities share common regulatory review practices that are critical in the establishment of a standardised review process for the GCC region. The differences identified in the five review processes were mainly due to the order of the steps carried out or the time spent in carrying out a certain procedure. Al-Essa (2011) showed that the approval timelines in the GCC states depend on the type of products being registered, the quality of the submitted data and the level of interaction between the sponsor and the authority.
Table 3.1
Number of approved products for GCC in 2008, 2009 and 2010
Countries | Type of company | 2008 | 2009 | 2010 |
|---|---|---|---|---|
Approved products | Approved products | Approved products | ||
Bahrain | Gulf | 5 | 5 | 51 |
Arab non-Gulf | 25 | 37 | 42 | |
Asian | 7 | 2 | 19 | |
International | 50 | 105 | 62 | |
Total | 87 | 149 | 174 | |
Kuwait | Gulf | 81 | 51 | 43 |
Arab non-Gulf | 94 | 38 | 46 | |
Asian | 17 | 3 | 5 | |
International | 224 | 106 | 116 | |
Total | 416 | 198 | 210 | |
Oman | Gulf | 52 | 37 | 56 |
Arab non-Gulf | 15 | 25 | 29 | |
Asian | 15 | 5 | 12 | |
International | 89 | 105 | 81 | |
Total | 171 | 172 | 178 | |
Qatar | Gulf | 56 | 57 | 134 |
Arab non-Gulf | 39 | 13 | 11 | |
Asian | 1 | 0 | 0 | |
International | 107 | 30 | 62 | |
Total | 203 | 100 | 207 | |
Saudi Arabia | Gulf | 150 | 91 | 16 |
Arab non-Gulf | 25 | 52 | 19 | |
Asian | 0 | 0 | 0 | |
International | 134 | 132 | 57 | |
Total | 309 | 275 | 92 |
In 2010, Kuwait registered the highest number amongst all the GCC countries with a total of 210 products registered, which is almost twice than that of Saudi Arabia (92 products) (Table 3.1).
Oman is the only country in the GCC with a constant number of products registered from 2008 to 2010. Bahrain saw a steady rise in the number of registered medicines from 87 approved products in 2008, 149 in 2009 and 174 in 2010 (Fig. 3.1). This steady increase in the registration of medicines in Bahrain was due to the decision from the Ministry of Health (MOH) to facilitate the procedures for the drug registration if the product is approved by advanced regulatory authorities such as the EMA and USFDA or by one of the reference countries in the Gulf. Kuwait, on the other hand, saw a decrease in the number of registered medicines in 2009 and 2010 of 198 and 210, respectively, as compared to 416 in 2008. According to the Kuwait regulatory authority, the high number of approvals in 2008 was attributed to the pending files with minimum requirement which were finalised in 2008, hence an increase in the total number of registered products in that year.