(1)
University of South Australia, Adelaide, Australia
Abstract
The first rule of the new drug reimbursement game is to recognise it is a game and that the regulator makes the rules. The economic expression of this game is the Political Economy of New Drugs (PEND). The global PEND is driven and shaped primarily by the US: its pharmaceutical industry; its government via trade-negotiations with the Organisation for Economic Cooperation and Development (OECD); and US-based academic pharma-economists and the evidence they generate. In this chapter I use the PEND to illustrate the characteristics of this game. What is the political economy of new drugs? How does it influence the research agenda? Does it change over time? As the US starts to address issues such as whether it should use evidence of cost-effectiveness to make decisions about drug reimbursement, the global PEND must adapt to respond to the new forms of evidence and decision rules. I demonstrate how OECD regulators outside the US could use this time of change to reframe the global PEND. The reframed PEND would facilitate a strategic and economically meaningful choice in the decision threshold and allows regulators to respond optimally to the primary strategy of the pharmaceutical industry; the threat that lowering the price below a firm’s preferred price is not in the best interests of the population.
2.1 The Political Economy of New Drugs
The term “Political Economy” is a former name of the discipline of economics. Today it is used in a number of senses, and its usage continues to evolve. Common to most of the modern interpretations is the economic analysis of tension in policy choices in a context that recognises both political and economic influences (Groenewegen 2008). In this book, the Political Economy of New Drugs (PEND) is defined following the precedent set by Comanor in his 1986 paper: “The Political Economy of the Pharmaceutical Industry”. While Comanor did not explicitly define his use of this term, it can be inferred that the political economy of the pharmaceutical industry concerns the economics of the critical choices governments need to make about the pharmaceutical industry and its regulation. He identifies economics as a “practical science” the practitioners of which have always been interested in the “critical choices” by governments.1
Of particular interest to Comanor was the relationship between the economist’s research agenda and the politics of pharmaceutical regulation. He found that the political economy framed the research, and as the political debate changed so too did the research.
In this book the focus is “the political economy of new drugs”: the factors that influence how any surplus associated with a new drug or a future drug is allocated across stakeholders, including consumers, purchasers, budget holders and firms.2 The relationship between the economic research agenda and the political process identified by Comanor is also a central issue in this book. This book’s focus on the political economy of new drugs rather than the pharmaceutical industry itself reflects the increased role of CEA in informing pricing and the capacity for industry, researchers and institutions to quantify the innovation associated with individual new drugs.
One way that the pharmaceutical industry seeks a share of a new drug’s surplus is through lobbying. Lobbying plays an important part in the allocation of surplus associated with patented innovation in any sector of the economy. In the case of new drugs, lobbying tends to focus on the question of an appropriate price for a new drug, given the health-generating potential of both that drug and of future innovation funded by sales of the drug. The associated policy choices include: (1) whether new drug price should be regulated; (2) the selection of a decision threshold price in a reimbursement process; and (3) whether bilateral Free Trade Agreements (FTAs) with the US should be used by the US to prevent partner countries from regulating the price US firms prefer for their new drugs.
In the broader economy, lobbying by patent-holding firms is characterised as rent seeking or rent protection.3 In the prevailing PEND, lobbying for higher new drug prices is instead characterised as providing incentives for investment into further R&D. This way of framing the impetus for lobbying links increased price to both increased profits and increased future health outcomes, thus creating an apparent win-win situation for firms and consumers. These claims of the relationship between new drug price and future health are supported by peer reviewed research and government studies (Comanor 1986; Scherer 2000; International Trade Administration 2004; Vernon et al. 2009). This evidence base supports the claim that the relationship between price and R&D investment is positive and that new drugs are a key driver of improved longevity (life expectancy) for consumers.4 The claim that firms rely on non-capital market-funded R&D rather than capital market borrowings due to the riskiness of this investment is supported by the pharma-economic literature.5 The claim of and evidence for a win-win outcome to the policy of higher prices for new drugs is critical to the success of lobbying by US Pharma.
2.2 The Rate of Return on Investment in Pharmaceutical R&D and the Political Economy
Comanor argued that the political economy of the pharmaceutical industry shaped the economic research agenda, most notably the premise that there is a trade-off between savings today and health tomorrow: society can have more of one and less of the other but not more of both. Hence the purpose of much of the research was to quantify this trade-off. Comanor noted that the literature did not question whether this trade-off exists. Instead the research agenda prioritised finding an estimate of this trade-off, in the form of the ratio of the return (future health) on the original investment. Comanor identified three potentially relevant rates of return: the return to the firm in terms of economic rent from their investments; the return to the industry overall; and the social return, where return is measured as the increase in social welfare (economic rent and consumer welfare) from the investment in higher prices.
Comanor found that the focus on evidence of these rates of return was the single issue common to the disparate economic literature on the pharmaceutical industry. He also found that, at the time of his review (1986), no reliable estimate of the social rate of return on R&D had been published in the peer reviewed literature.6 Comanor concluded that it could be possible to increase competition (lower price) without having a loss in future innovation, however the current political economy excluded this possibility from the research agenda. Consequently, the evidence that could test this hypothesis (the possibility that there is no trade-off) was not available.
2.3 Is the Political Economy of New Drugs Constant?
Comanor observed that during the period from 1959 to 1985, the political economy of the pharmaceutical industry was reframed at least twice in response to changes in the political debate. The focus shifted from questioning whether the industry did in fact experience monopoly rents, to accepting that they did and then considering the impact of regulation on these rents and the incentive for R&D. Comanor also noted that the adversarial nature of the political debate was reflected in the economic research. He notes that at the start of economists’ engagement with this political economy the focus was not on identifying critical trade-offs. Instead each side of the debate had a different premise, specifically, that the key issue was that competition should be restricted in order to maximise innovation and hence social welfare and the second ignoring this relationship.7
In the 25 years since Comanor’s 1986 review of the political economy of the US pharmaceutical industry, the following have continued to grow: the US pharmaceutical industry8; US expenditure on pharmaceuticals and health as a percentage of Gross Domestic Product (GDP)9; the number of new drugs in the US development pipeline10; and the average longevity of the US population.11 Studies have provided further evidence that the following relationships are positive: new drug price and R&D investment by firms (Vernon 2005); R&D investments and new drugs, as summarised by the costs of bring a new drug to market (DiMasi 2001); and new drugs and longevity (Lichtenberg 2006). Furthermore, other evidence suggests that the costs of bringing a new drug to market continues to increase as does society’s demand for new drugs, particularly in relation to chronic diseases for which obesity is a risk factor (Grabowski et al. 2002; DiMasi et al. 2003).12 The evidence supporting the case for higher new drug price appears to have strengthened, but the focus of evidence development has not broadened; the landscape of this political economy appears to have intensified but not shifted.
Since 1986, there have also been three main developments in the global pharmaceutical economy. First, institutions throughout the OECD started using formal processes such as HTA/CEA13 to assess the incremental costs and benefits of new drugs compared with the best existing therapy.14 The results of HTA/CEA are then used in conjunction with a decision threshold and other information to assess whether the population will be better or worse off if the institution reimbursed the drug at the firm’s offer price. Hence, the policy debate throughout much of the OECD is increasingly broader than that of the US debate. The latter is primarily concerned with policies around discounts to large purchasers, whether there should be universal access to drugs, and whether HTA/CEA should be used and prices regulated. The rest of the OECD is additionally concerned with the choice of decision threshold and the type of information that should be included in an assessment of costs and benefits of new drugs.15 However, the imperative to maximise the benefits of pharmaceutical and biotechnology innovation remains a significant part of OECD-wide research on pharmaceutical policy.16
Second, there has been a significant increase in the quantity of evidence about the relationship between: (1) price and innovation; and (2) new drugs in general and health.17 However, it was only comparatively recently that the US pharma-economic literature provided two estimates of the ratio of the social return on consumers’ investment in higher drug prices in the US. Lichtenberg’s 2004 estimate of the social return on additional investment in new drug R&D is in the order of 160:1. Santerre and Vernon’s (2006) estimate of a return on consumer’s investment via higher prices over the period 1960–2000, in terms of the value of the additional health benefits from additional drugs, is in the order of 28:1.
Third, the US pharmaceutical industry now has two additional avenues to take the PEND to the rest of the OECD: (1) the formal reimbursement process for individual new drugs (lobbying for choice of decision threshold) (Vernon et al. 2010); and (2) the bilateral FTAs between the US and OECD countries (lobbying to prevent trading partners from regulating new drug price) (Harvey et al. 2004).
US pharma-economists have sought to adapt the original US political economy and research agenda to accommodate some of these changes. For example, Vernon et al. (2009) chose to define the socially optimal threshold from the perspective of optimal innovation. The authors started with the premise that socially optimal decision investment in R&D occurs when the firm can appropriate 100 % of the associated social surplus. Vernon et al. argue that this result occurs when the incremental cost per quality-adjusted life year (QALY) of a new drug is the same as the incremental cost per QALY of the least cost-effective of currently funded services. The authors argue this reference is the provision of dialysis at a cost per QALY of $129,000. Other authors have argued that setting a price threshold of i 18 and comparing the results of CEA against this threshold of i is price control under another name and its result is the same: pricing below the free market price will lead to a deadweight social loss.19 Jena and Philipson have published a number of papers about the inclusion of dynamic welfare considerations in the decision threshold (Jena and Philipson 2007, 2008). Originally they argued that this threshold should be the maxWTP, just as Vernon et al. have claimed. Their rationale included that “technology adoption through cost-effectiveness is a price-control policy in disguise and might therefore have many of the properties of such policies.” However, in the later paper they recognised a number of factors that supported the case for the threshold to be lower than the maxWTP. These factors include budget constraints and the contribution by public sector research funds to pharmaceutical R&D. Jena and Philipson did not specify exactly what this price should be, only that it should be higher than the threshold applied to non-pharmacological therapies.
One key aspect of the political economy has remained constant, despite these developments: the trade-off between savings today and health tomorrow remains the central premise.20 The possibility that increased competition (lower prices) could lead to more health in the future as well as today is not part of the research agenda. Furthermore, it is a possibility that continues to be excluded from the prevailing political economy.
2.4 Reframing the Political Economy
Is it possible to reframe rather than adapt the prevailing PEND to accommodate the developments in drug reimbursement and HTA/CEA?
Could this reframed political economy include the possibility that each of the following can be simultaneously improved: competition; current health; and future health?
This reframed political economy would focus on the central policy decision by a reimbursing institution: which decision threshold will maximise the npvPH? The first step in this research was to develop a formal model to define the political economy of new drugs in the context of policy choice and research. The model was used to specify both the current and alternative frames for the political economy.
2.4.1 Architecture of Evidence Based Policy
The relationship between the PEND and the research agenda is characterised using an adaption of Grüne-Yanoff and Schweinzer’s Architecture of Game Theory (Grüne-Yanoff and Schweinzer 2008) with additional elements derived from Roe (1991) and Comanor (1986). The adaption is described in Pekarsky (2012, Appendix 1) and illustrated in Fig. 2.1. Amongst other advantages, this framework identifies the line of reasoning that leads to certain possibilities being excluded from the prevailing research agenda. For example, by defining the key trade-off as being between more health tomorrow and more savings today, the possibility that both competition and population health can be improved is excluded. Consequently, this framework identifies that there is a requirement to redefine the evidence based policy framework that shapes the current research agenda and suggests some mechanisms by which this could be achieved.
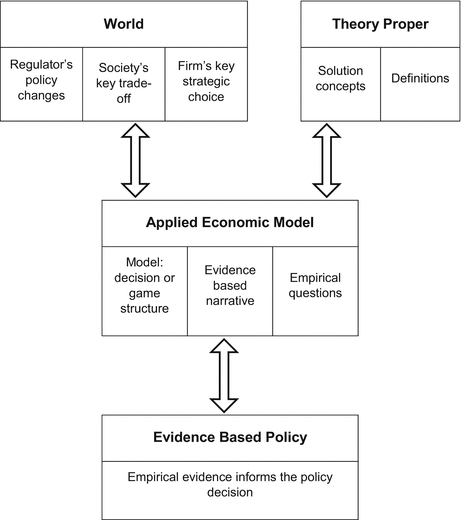

Fig. 2.1
An architecture of applied economics
The following two sections populate this framework, first with a characterisation of the prevailing political economy and the second with an alternative frame.
2.4.2 Prevailing Political Economy
The key trade-off is between savings today and health tomorrow, for example:
If the price of today’s new drug is reduced below the FPP, there will be financial savings for some today but this is at the cost of access to more drugs for the whole population in the future.21
The key decision by the firm is how much to invest in R&D and the key policy choice is whether or not to regulate or control the new drug price. This particular framing inspires research questions such as:
This frame excludes empirical questions about the direction of the relationship between: (1) new drug price, R&D, number of new drugs; and (2) future health of the population. This relationship is assumed to be positive under all conditions. The critical piece of information that will inform the regulator is the return on this investment in R&D (financed via higher prices and public investment), where this return is measured as the additional health gains possible from the availability of additional drugs in the future. If the health effects are monetised, for example using an estimate of the value of an additional year of life, then the return can be compared to the investment as a ratio. If this ratio is high then increased prices today represent a good evidence-based policy choice. If this ratio is less than one, then there is a net loss on the original investment.
The evidence-based policy narrative takes the following or a related form.
New drugs have been shown to be the key driver of historic gains in life expectancy for the US population. In order to achieve sustained increases in life expectancy, more new drugs are needed in the future. Pharmaceutical innovation is driven by R&D investments by firms. R&D investments are driven by higher new drug prices, acting as both an incentive and a funding source for ongoing R&D. The value of the possible health gains far outweigh the financial costs of R&D, therefore higher—unregulated—prices represent good policy.
2.4.3 An Alternative Political Economy of New Drugs
This book explores the fresh paths for research and different critical research questions opened by reframing the prevailing PEND. There are many ways that the political economy could be reframed. The frame used in this book is summarised as follows.
The key trade-off is between savings for health purchasers today and firms’ profits. The evidence for this trade-off is twofold. First, a firm would not lobby for a higher price unless this strategy increased its profits in the current period. Second, an institution would not reject a higher price of new drugs if it also decreased costs of providing the same health benefits from today’s budget. Therefore the existence of this trade-off is a reasonable premise.
The key decision by the firm is how to maximise profits today and tomorrow. One strategy available to the firm is to minimise the R&D costs borne by the firm by creating an incentive for institutions to subsidise these costs. One mechanism by which this is achieved is to increase the price of current drugs, without reducing quantity sold (for example, increase the decision threshold). The key policy choices for the institution are: (1) what should the decision threshold for the health effects for new drugs be; and (2) should this threshold be altered, given that there is a relationship between new drug price today and future population health. This particular framing inspires research questions such as:
Given that budgets are constrained or fixed, under what conditions will increased pharmaceutical R&D today necessarily lead to increased population health in the future?;
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


