Refractory Cytopenia with Multilineage Dysplasia
Carla S. Wilson, MD, PhD
Key Facts
Terminology
MDS with cytopenia(s) and dysplasia
Cytopenia in 1 or more lineages
Dysplasia in 2 or more lineages
Blasts
< 1% in peripheral blood, < 5% in bone marrow
Account for 30-40% of MDS
Etiology/Pathogenesis
Clonal expansion of a neoplastic hematopoietic stem/progenitor cell
Clinical Issues
Patients commonly seek medical attention for symptoms of anemia
Microscopic Pathology
Dysplasia
Bone marrow
Typically hypercellular
Abnormal architecture in biopsy sections
Ancillary Tests
Perform CD34 immunostains on biopsy sections
If aggregates (3-5 cells) or clusters (> 5 cells) of CD34 positive blasts, consider RAEB instead
Cytogenetic analysis
50% have abnormal karyotypes
˜ 20% have unfavorable karyotypes
Top Differential Diagnoses
Therapy-related myeloid neoplasms
Diagnostic Checklist
Former category of RCMD-RS is now incorporated into RCMD
TERMINOLOGY
Abbreviations
Myelodysplastic syndrome (MDS)
Refractory cytopenia with multilineage dysplasia (RCMD)
Synonyms
RCMD-ring sideroblasts (RS)
Definitions
MDS with cytopenia(s) and dysplasia
1 or more cytopenias
Anemia, neutropenia, &/or thrombocytopenia
Dysplasia in 2 or more myeloid lineages
Erythroid, granulocytic, and megakaryocytic
Blasts: < 1% in peripheral blood, < 5% in bone marrow
Includes the former category of RCMD-RS
ETIOLOGY/PATHOGENESIS
Environmental Exposure
Benzene and other industrial solvents
Insecticides, pesticides
Cigarette smoking
Pathogenesis
Clonal expansion of a neoplastic hematopoietic stem/progenitor cell
Gene mutations
Maintain proliferation of early progenitor cells
Abnormal cell maturation
Proportional loss of mature cells
Progressive decrease in normal hematopoietic stem/progenitor cells
Role for tumor suppressor genes in disease onset
Abnormal T-cell mediated immune dysregulation
Secondary B-cell immune dysregulation
Abnormal marrow microenvironment
Ineffective hematopoiesis
Peripheral cytopenias
Increased apoptosis of late marrow precursors
CLINICAL ISSUES
Epidemiology
Incidence
Accounts for 30-40% of MDS
Age
Disease of older individuals
Peak incidence for men is 70-74 years
Peak incidence for women is 75-79 years
Gender
Incidence is slightly higher in men
Presentation
Symptoms relate to cytopenias
Commonly seek medical attention for symptoms of anemia
Fatigue, dyspnea, palpitations, headache, dizziness
Exaggerated bleeding
Thrombocytopenia and platelet dysfunction
Infection
Neutropenia and neutrophil dysfunction
Occasional mild hepatomegaly or splenomegaly
Laboratory Tests
Complete blood count and differential cell count
Degree of cytopenia required unless definitive morphologic or cytogenetic abnormalities
Hemoglobin < 10g/dL
Neutrophil count < 1.8 × 109/L
Platelet count < 100 × 109/L
Bone marrow evaluation
Cytogenetic evaluation is essential
Treatment
Options, risks, complications
Therapeutic considerations are based on the following factors
Age and comorbidities, risk category
Higher risk disease in patients who cannot tolerate high-intensity therapy
Supportive care alone, new therapeutic agents
Drugs
Immunomodulatory drugs
Methyltransferase inhibitors
Azacytidine and decitabine
Prolongs survival in intermediate- to high-risk patients
Allogeneic hematopoietic stem cell transplantation
Only curative treatment
Reduced intensity conditioning regimens reduce morbidity
Prognosis
Majority of patients have intermediate IPSS risk score
Pathologic determinants of prognosis include
Type of karyotypic abnormalities
Complex karyotype or -7 are poor prognostic markers
Degree of cytopenias or dysplasia
Presence of circulating blasts and blast % in bone marrow
Overall median survival is 30-60 months
˜ 10% develop AML at 2 years after diagnosis
Patients with complex karyotypes have similar survivals to patients with refractory anemia with excess blasts (RAEB)
MICROSCOPIC PATHOLOGY
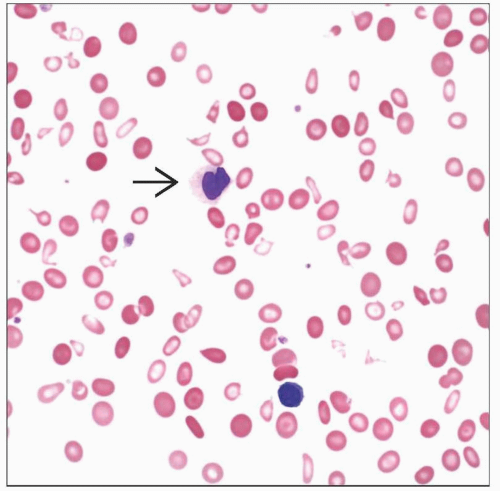
Peripheral Blood
Unicytopenia or bicytopenia
Anemia is often macrocytic
Reduced polychromasia
Dysplasia
May need buffy coat if markedly leukopenic
< 1% circulating blasts
No monocytosis or pancytopenia
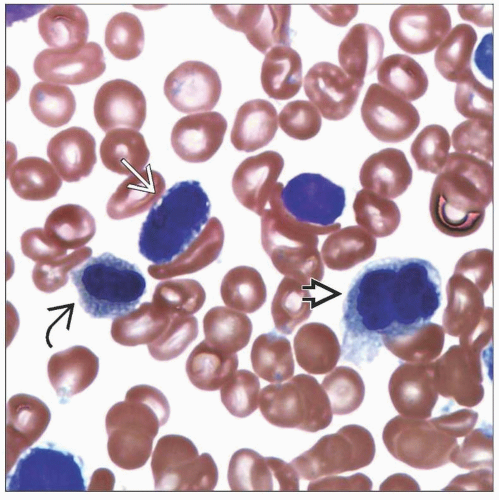
Bone Marrow
Typically hypercellular
≥ 10% dysplasia in 2 or 3 lineages
Megakaryocyte dysplasia is based on assessment of ≥ 30 megakaryocytes in biopsy sections
Some studies suggest > 40% megakaryocytic dysplasia is better criterion than ≥ 10%
< 5% blasts
Abnormal architecture in biopsy sections
Abnormal localization of immature precursors (ALIP)
Abnormal paratrabecular location of megakaryocytes or erythroid precursors
Increased megakaryocytes with clustering
Disruption of erythroid colonies
Subset of cases may be hypocellular or fibrotic
Histiocytes often increased
Prussian blue iron stain
Evaluate aspirate smear or touch preparation
Increased stores &/or erythroid iron incorporation
Increased erythroid iron incorporation
Ring sideroblasts may comprise ≥ 15% of erythroid precursors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree