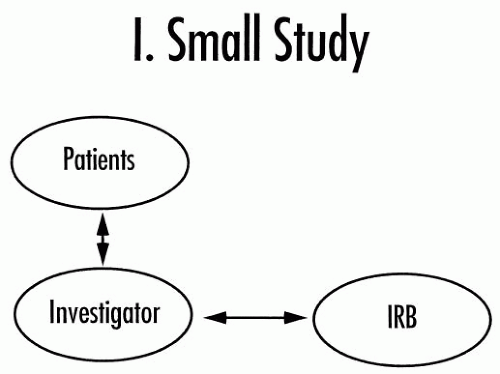
academic investigator has a large staff, including competent subinvestigators, it may be possible and potentially desirable for both parties to have some or most of the work delegated.
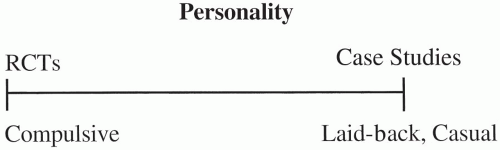 Figure 80.1 Spectrum of personalities and the types of trials best suited for each. RCTs, randomized controlled trials. |
practice physicians who feel that the patient’s interests come first and cannot be compromised, even in the interests of adhering to the protocol used in a clinical trial. Some of the primary motivations of pharmaceutical sponsors in choosing investigators and sites are shown in Table 80.1.
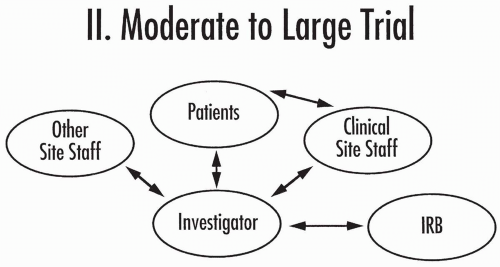 Figure 80.3 Core organization of a moderate to large clinical trial. IRB, Institutional Review Board. |
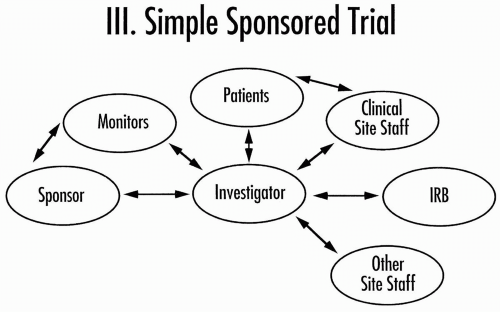 Figure 80.4 Core organization of a simple clinical trial sponsored by a pharmaceutical company. IRB, Institutional Review Board. |
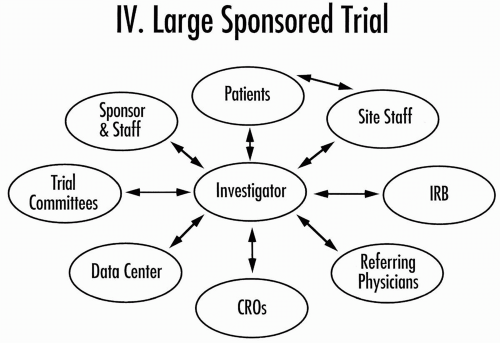 Figure 80.5 Core organization of a large and more complex sponsored clinical trial. IRB, Institutional Review Board. |
Table 80.1 Some of the primary motivations for a sponsor when choosing an investigator and site for a clinical trial | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 80.2 Concerns of investigators in an academic environment or in private practice regarding their participation in industry-sponsored clinical trials | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
trials well. Unique patient populations, facilities, and equipment are also important to bring to the attention of potential sponsors. A brochure that can be widely disseminated as well as placed on the institution’s website is one of many means of publicizing the institution’s capabilities.
Table 80.3 Investigational New Drug Application/Investigational Device Exemptions services provided to faculty researchers by the Investigational New Drug Application/Investigational Device Exemption Assistance Program, Academic Health Center, University of Minnesotaa
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|