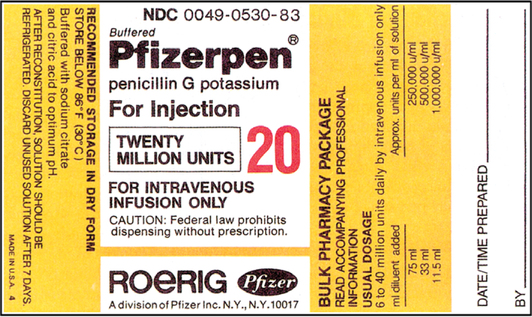
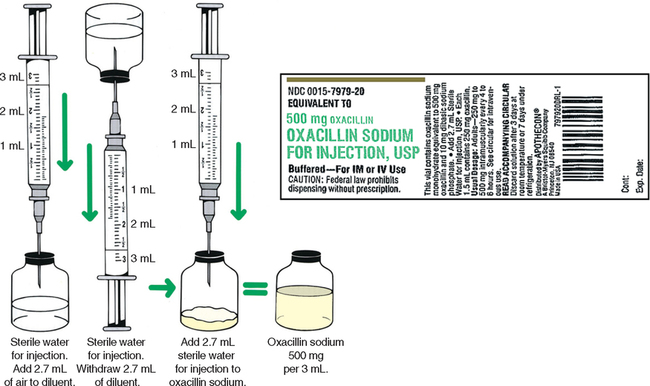
CHAPTER 19 After reviewing this chapter, you should be able to: 1. Prepare a solution from a powdered medication according to directions on the vial or other resources 2. Identify essential information to be placed on the vial of a medication after it is reconstituted 3. Determine the best concentration strength for medications ordered when there are several directions for mixing 4. Identify the varying directions for reconstitution and select the correct directions to prepare the dosage ordered 5. Calculate dosages from reconstituted medications 6. Determine the rate in milliliters per hour for enteral feedings 7. Calculate the amount of solute and solvent needed to prepare a desired strength for enteral feedings • Solute—a powdered medication or liquid concentrate to be dissolved or diluted. • Solvent (diluent)—a liquid that is added to the powder or liquid concentrate. The nurse must identify the solvent (diluent) to use. The type of solvent (diluent) varies according to the medication. The package insert or the medication label will indicate the solvent (diluent) to be used and the amount. If the information is not indicated on the label or the package insert is unavailable consult the pharmacy or a medication reference such as the Physician’s Desk Reference (PDR), a medication reference, or the hospital formulary. • Solution—the liquid that results when the solvent (diluent) dissolves the solute (powdered medication or liquid concentrate). 1. The drug manufacturer provides directions for reconstitution, including information regarding the number of milliliters of diluent or solvent that should be added, as well as the type of solution that should be used to reconstitute the medication. The concentration (or strength) of the medication after it has been reconstituted according to the directions is also indicated on some medications. The directions for reconstitution must be read carefully and followed. 2. The diluent (solvent, liquid) commonly used for reconstitution is sterile water or sterile normal saline solution, prepared for injection. Other solutions that may be used are 5% dextrose and water and bacteriostatic water. Some powdered medications for oral use may be reconstituted with tap water. The manufacturer’s directions will tell you which solution to use. If the medication requires a special solution for reconstitution, it is usually supplied by the drug manufacturer and packaged with the medication (e.g., Librium). 3. Once you have located the reconstitution directions on the label, you need to identify the following information: a. The type of diluent to use for reconstitution. b. The amount of diluent to add. This is essential because directions relating to the amount can vary according to the route of administration. There may be different dilution instructions for intravenous (IV) versus intramuscular (IM) administration. c. The length of time the medication is good once it is reconstituted. The length of time a medication can be stored once reconstituted can vary depending on how it is stored. When medications are reconstituted the solution must be used in a timely fashion. The potency (stability) of the medication may be several hours to several days or a week. Check the medication label, package insert, or appropriate resources for how long a medication may be used after reconstitution. d. Directions for storing the medication after mixing. Medications must be stored appropriately once reconstituted per manufacturer’s instructions to ensure optimal potency of the medication. Medications can become unstable when stored incorrectly and for long periods. Example: A label may state a medication maintains its potency 96 hours at room temperature or 7 days when refrigerated. e. The strength or concentration of the medication after it has been reconstituted. Refer to Figure 19-1 showing the oxacillin sodium reconstitution procedure. Note that directions on the label say to add 2.7 mL sterile water for injection and that each 1.5 mL contains 250 mg. The available dosage after reconstitution is 250 mg of oxacillin per 1.5 mL solution (500 mg per 3 mL). 4. If there are no directions for reconstitution on the label or on a package insert, or if any of the information (listed in number 3) is missing, consult appropriate resources such as the Physician’s Desk Reference (PDR), a pharmacology text, the hospital drug formulary or the pharmacy. 5. Injectable medications for reconstitution can come in a single-dose vial or a multiple-dose vial. When medications are in single-dose vials, there is only enough medication for one dose and the contents are administered after reconstitution. In the case where the nurse reconstitutes a multiple-dose vial, there is enough medication for more than one dose. Therefore, when a multiple-dose vial is reconstituted, it is important to clearly label the vial after reconstitution with the following information: a. The date and time prepared, dosage strength prepared, the expiration date for the medication once reconstituted, and the time. Note: If all of the solution that is mixed is used, this information is not necessary. Information regarding the date and time of preparation and date and time of expiration is crucial when all of the medication is not used. b. Storage directions such as “Refrigerate.” 6. When reconstituting medications that are in multiple-dose vials or have several directions for preparation, information regarding the dosage strength or final concentration (what the medication’s strength or concentration is after you mixed it) must be on the label; for example, 500 mg per mL. This is important because others using the medication after you need this information to determine the dosage. 7. After the diluent is added to a powder, some medications completely dissolve and there is no additional volume added. Often, however, the powdered medication adds volume to the solution. The powdered medication takes up space as it dissolves and results in an increase in the amount of total (fluid) volume once it has dissolved. This is sometimes referred to as the displacement factor, or just displacement. The reconstituted material represents the diluent and powder. For example, directions for 1 g of powdered medication may state to add 2.5 mL sterile water for injection to provide an approximate volume of 3 mL (330 mg per mL). When the 2.5 mL of diluent is added, the 1 g of powdered medication displaces an additional 0.5 mL, for a total volume of 3 mL. The available dosage after reconstitution is 330 mg per milliliter of solution. The two types of reconstituted parenteral solutions are single strength and multiple strength. A single-strength solution has the directions for reconstitution printed on the label, as shown on the 500-mg oxacillin label in Figure 19-1. A multiple-strength solution usually has several directions for reconstitution and requires the nurse be even more attentive to the directions to select the best concentration to administer the required dosage. Let’s do some practice problems answering questions relating to single-strength solutions. 1. Consider the route of administration. a. IM—You are concerned that the amount does not exceed the maximum allowed for IM administration. However, you do not want to choose a concentration that will result in irritation when injected into a muscle. When a choice of strengths can be made, do not choose an amount that would exceed the amount allowed for IM administration or one that is very concentrated. b. IV—Keep in mind that this medication is usually further diluted because once reconstituted, the medication is then placed in additional fluid of 50 to 100 mL or more, depending on the medication being administered. Example: Erythromycin requires that the reconstituted solution be placed in 250 mL of fluid before administration to a client. In pediatrics a medication may be given in a smaller volume of fluid, depending on the child’s age, the child’s size, and the medication. 2. Choose the concentration or dosage strength that comes closest to what the prescriber has ordered. The dosage strengths are given for the amount of diluent used. Example: If the prescriber orders 300,000 units of a particular medication IM, and the choices of strength are 200,000 units per mL, 250,000 units per mL, and 500,000 units per mL, the strength closest to 300,000 units per mL is 250,000 units per mL. It allows you to administer a dosage within the range allowed for IM administration, and it is not the most concentrated. 3. The word respectively may sometimes be used on a medication label for directions on reconstitution. For example, reconstitute with 23 mL, 18 mL, 8 mL of diluent to provide concentrations of 200,000 units per mL, 250,000 units per mL, 500,000 units per mL, respectively. The word respectively means in the order given. In terms of the directions for reconstitution, this means that if you add 23 mL diluent, it will provide 200,000 units per mL, 18 mL diluent will provide 250,000 units per mL, etc. In other words, the amounts of diluent correspond to the order in which the concentrations are written. Remember: When you are mixing a medication that is a multiple-strength solution, the dosage strength that you prepare must be written on the vial. Let’s look at a sample label that shows a multiple-strength solution.
Reconstitution of Solutions
BASIC PRINCIPLES FOR RECONSTITUTION
RECONSTITUTING MEDICATIONS WITH MORE THAN ONE DIRECTION FOR MIXING (MULTIPLE STRENGTH)
Guidelines for Choosing Appropriate Concentrations

Reconstitution of Solutions

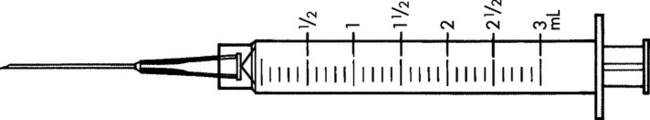
 Practice Problems
Practice Problems

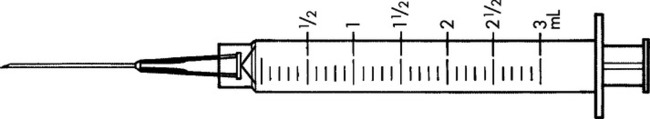

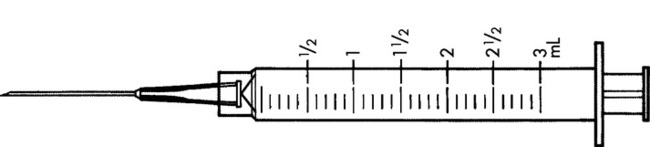

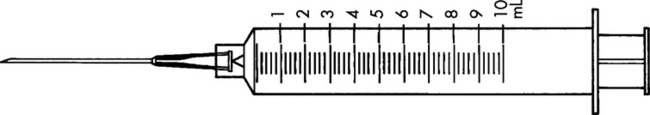


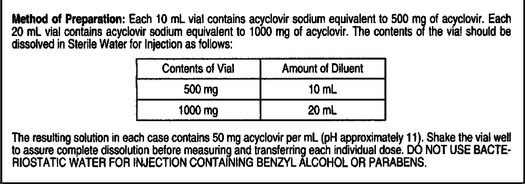


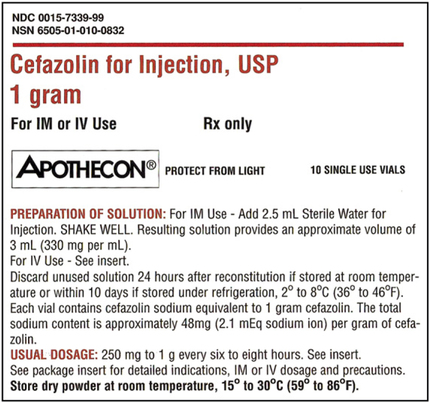

 Practice Problems
Practice Problems