Fig. 1
Schematic diagram of origins of PAR ligands in the skin. Figure shows cross section through the skin. The proteases originate from endogenous sources including keratinocytes and immune cells as well as from external sources, such as plants
Whether the activation of PAR-2 contributes to initiating itch in mice remains controversial. SLIGRL- and trypsin-evoked scratching remained in PAR-2 knockout mice (Liu et al. 2011). In contrast, tryptase-evoked scratching was inhibited by genetic knockout of PAR-2 as well as pharmacological blockade of PAR-2 (Ui et al. 2006). Different types of agonists (e.g., SLIGRL, tryptase, and trypsin) may activate different signaling pathways. Alternatively, roles of PAR-2 may be different between neurons and keratinocytes.
PARs are involved in pathological conditions accompanied by chronic itch. The number of tryptase-containing mast cells in the upper dermis is increased in atopic dermatitis as well as psoriatic skin (Harvima et al. 1990; Jarvikallio et al. 1997). Mutations in the serine protease inhibitor Kazal type 5 (spink5) gene, which encodes the protease inhibitor lymphoepithelial Kazal-type-related inhibitor, result in upregulation of KLK5 activity which is involved in the formation of atopic dermatitis-like skin lesions via PAR-2 (Briot et al. 2009) (Table 1). Consistent with this, transgenic KLK5 overexpressor mice displayed signs of severe inflammation and pruritus (Furio et al. 2014). Cathepsin S is mainly present in the dermis of normal human skin. In contrast, cathepsin S is upregulated and detected in keratinocytes in psoriatic skin (Schonefuss et al. 2010). Overexpression of cathepsin S induces atopic dermatitis-like skin through an increase in PAR-2 expression in dendritic cells (Kim et al. 2012). Transgenic expression of the serine protease channel-activating protease-1 in the skin induces atopic dermatitis-like skin accompanied by increased spontaneous scratching (Frateschi et al. 2011). These phenotypes are completely negated when superimposed on a PAR-2 null background. In the skin of NC mice, an animal model of atopic dermatitis, the activity of serine proteases as well as the number of PAR-2-positive keratinocytes is increased (Tsujii et al. 2009). These findings imply that PARs play an important role in chronic itch under pathological conditions.
Table 1
Protease-related mutant mice with atopic dermatitis-like skin
Mutations | Upregulated proteases | Subtype of PARs | References |
|---|---|---|---|
Serine protease inhibitor Kazal type 5 | KLK5 | PAR-2 | Briot et al. (2009) |
KLK5 | KLK5 | Not tested | Furio et al. (2014) |
Cathepsin S | Cathepsin S | PAR-2 | Kim et al. (2012) |
Serine protease channel-activating protease-1 | Serine protease channel-activating protease-1 | PAR-2 | Frateschi et al. (2011) |
2 Mechanisms of Protease-Activated Receptor-Mediated Itch
PARs are expressed broadly in neuronal as well as nonneuronal cells (Shpacovitch et al. 2002, 2007; Steinhoff et al. 2003; Zhu et al. 2005; Moormann et al. 2006; Vellani et al. 2010). The activation of PAR-2 on keratinocytes induces the release of LTB4 (Zhu et al. 2009b), which elicits itch through BLT1-expressing neurons (Andoh and Kuraishi 1998, 2005). Activation of PAR-2 in keratinocytes also induces release of thymic stromal lymphopoietin, a pruritogenic cytokine that excites TRPA1-expressing sensory neurons (Wilson et al. 2013). PARs are expressed by a variety of different immune cells, including neutrophils, eosinophils, monocytes, macrophages, and mast cells (Shpacovitch et al. 2007). In particular, PAR-1 is expressed in mast cells. Considering the partial inhibition of the PAR-1 agonist-evoked scratching by an H1 histamine receptor antagonist (Tsujii et al. 2008), PAR-1 agonist-evoked itch can be partially attributed to histamine released from mast cells. Although PAR-2 and PAR-4 are expressed by mast cells, an intradermal injection of PAR-2 or PAR-4 agonist presumably does not activate these receptors expressed by mast cells, since H1 histamine receptor antagonists failed to inhibit scratching evoked by the PAR agonists (Tsujii et al. 2008; Akiyama et al. 2012a). In addition to mast cells, other immune cells may be activated through PARs under pathophysiological conditions to release certain pruritogens. PAR-1, PAR-2, and PAR-4 have been found to be expressed in primary sensory neurons (Steinhoff et al. 2003; Zhu et al. 2005; Vellani et al. 2010). Proteases such as trypsin and thrombin and hexapeptide ligands of PAR-1, PAR-2, and PAR-4 can activate primary sensory neurons (Amadesi et al. 2004; Akiyama et al. 2010a; Vellani et al. 2010). Direct activation of primary sensory neurons through these receptors might contribute to itch. A PAR-2 agonist and either a PAR-1 or PAR-4 agonist apparently activate different subpopulations of primary sensory neurons (Vellani et al. 2010).
The neuronal pathway for PAR-mediated itch has been studied mainly using cowhage and PAR-2 tethered ligands such as SLIGRL. Itch elicited by intradermal insertion of cowhage spicules is considered to be non-histaminergic based on the following observations. (1) In contrast to histamine, cowhage spicules elicit itch without accompanying flare (Johanek et al. 2007; Sikand et al. 2009). (2) While histamine-elicited itch is described as mosquito bite-like, cowhage-elicited itch is described as stinging, sharp, and prickly (Kosteletzky et al. 2009). (3) Desensitization of the skin with topical capsaicin abolished cowhage-induced itch but not histamine-induced itch (Johanek et al. 2007). (4) Cowhage-evoked itch was not inhibited by pretreatment with an H1 histamine receptor antagonist (Johanek et al. 2007). Itch elicited by cowhage spicules is apparently mediated by populations of C- as well as Aδ-fibers that are distinct from those mediating histamine-elicited itch. Mechano-insensitive C-fibers preferentially respond to histamine but not cowhage (Schmelz et al. 1997; Namer et al. 2008) (Fig. 2). In contrast, mechanosensitive, polymodal C-fibers readily responded to cowhage with lesser or no responses to histamine (Johanek et al. 2008; Namer et al. 2008). Mechanosensitive A-fibers also responded more vigorously to cowhage than to histamine, but some exclusively responded to histamine (Ringkamp et al. 2011). At the level of the spinal cord, cowhage and histamine activated separate subpopulations of primate spinothalamic tract neurons (Davidson et al. 2007, 2012). In the mouse, a majority of spinal neurons were activated by both SLIGRL and histamine (Akiyama et al. 2009a, b). This difference between murine and primate spinal neurons might be due to the agonistic activity of SLIGRL on Mas-related G-protein-coupled receptors C11 (MrgprC11) (Liu et al. 2011). In the brain, cowhage and histamine activated largely overlapping areas including thalamus, primary and secondary somatosensory cortices, posterior parietal cortex, superior and middle temporal cortices, PCC, ACC, precuneus, and cuneus. However, some areas exhibited more extensive activation by cowhage, including the insular cortex, claustrum, basal ganglia, putamen, thalamic nuclei, and pulvinar (Papoiu et al. 2012).
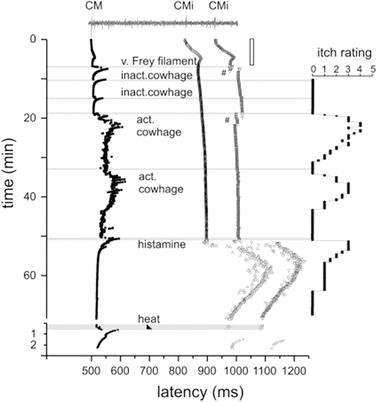

Fig. 2
Specimen of a multifiber recording from 1 mechano-responsive (CM) and 2 mechano-insensitive nociceptors (CMi). A trace of the raw signal containing the C-fiber action potentials is shown on top. Conduction latencies of these three marked fibers (filled square, open triangle) in response to successive electrical stimulation at the receptive field are plotted from top to bottom. Top traces were recorded during stimulation with increasing frequencies (see open square on right side), followed by traces recorded during stimulation with mechanical stimuli (v. Frey filament), inactive (inact.) and active (act.). Adapted from Namer et al. (2008)
The downstream signal transduction molecules involved in PAR-mediated itch have not yet been specified. Histaminergic itch requires TRPV1, whereas non-histaminergic itch via MrgprA3 and MrgprC11 agonists requires TRPA1 (Shim et al. 2007; Imamachi et al. 2009; Wilson et al. 2011). PAR-1-mediated itch may require TRPV1, but it is not currently known if itch mediated by PAR-2 and PAR-4 requires TRPV1 or TRPA1. Scratching evoked by trypsin, which acts at PAR-1, PAR-2, and PAR-4, was reduced in knockout mice lacking TRPV1 (Costa et al. 2008). Trypsin-evoked scratching was inhibited by an H1 histamine receptor antagonist as well as depletion of mast cells by repeated treatment with compound 48/80 (Costa et al. 2008), suggesting that mast cells play a major role in itch evoked by trypsin. Knockout mice lacking PAR-2 exhibited greater scratching compared to wild types (Liu et al. 2011), implying that PAR-2 is not involved in trypsin-evoked scratching. Considering that PAR-1 agonist-evoked scratching was partially inhibited by the H1 histamine antagonist, trypsin-evoked scratching is presumably mediated by PAR-1. It would be interesting to know whether scratching evoked by the PAR-2 and PAR-4 agonists requires TRPV1 or TRPA1. Phospholipase C (PLC) plays a key role in intracellular signaling by G-protein-coupled receptors. While PLCβ3 contributes to itch evoked by histamine and 5-HT, PLCβ3 does not appear to be involved in SLIGRL-evoked itch (Imamachi et al. 2009). Pirt (phosphoinositide-interacting protein) binds to phosphatidylinositol (4,5)-bisphosphate, TRPV1, and other ion channels to potentiate them. Knockout mice lacking Pirt exhibited a significant loss of scratching evoked by histamine, 5-HT, endothelin-1, and the MrgprA3 agonist chloroquine (Patel et al. 2011). On the other hand, SLIGRL-evoked scratching was not significantly inhibited in Pirt knockout mice.
3 Itch Sensitization via Protease-Activated Receptors
PARs are involved in sensitization of itch, with the features of spontaneous itch, hyperknesis (enhanced itch to a normally itchy stimulus), and alloknesis (itch elicited by an innocuous touch stimulus). These manifestations of itch sensitization are observed in patients suffering from chronic itch (Schmelz et al. 2003; Ikoma et al. 2004; Hosogi et al. 2006). In mice, hyperknesis has been demonstrated in a model of chronic dry skin itch. We reported that mice treated with drying agents exhibited significantly greater scratching following intradermal injections of 5-HT and SLIGRL delivered in the dry skin treatment area compared to control (water-) treated mice (Akiyama et al. 2010a). In contrast, histamine-evoked scratching was not significantly enhanced in dry skin-treated mice. DRG cells taken from the dry skin-treated mice exhibited significantly greater responses to 5-HT and SLIGRL, but not histamine, consistent with the behavioral results (Fig. 3). Superficial dorsal horn neurons receiving afferent input from a dry skin-treated hind paw exhibited significantly enhanced responses to SLIGRL, but not histamine, compared to units recorded in control animals (Akiyama et al. 2011). These findings suggest that the enhanced response to SLIGRL may be attributed to peripheral sensitization of pruriceptors projecting to the recorded dorsal horn neurons. NGF might account for this peripheral sensitization. In dry skin, NGF levels are elevated and might contribute to peripheral sensitization of pruriceptors (Tominaga et al. 2007). Intradermally administered NGF enhanced itch induced by cowhage but not histamine in humans (Rukwied et al. 2013) (Fig. 4). PAR-2 may be sensitized in the skin in which NGF levels are elevated under chronic itch conditions. Moreover, not only may pruriceptors expressing PARs become sensitized, but PARs may also contribute to sensitization of non-histaminergic pruriceptors expressing transduction molecules such as MrgprA3 and MrgprC11. Pretreatment with SLIGRL, but not BAM8-22, resulted in an enhancement of responses of primary sensory neurons to MrgprA3 and MrgprC11 agonists, as well as enhanced scratching evoked by the MrgprA3 and MrgprC11 agonists (Akiyama et al. 2012b). Pretreatment with SLIGRL failed to enhance scratching evoked by histamine, 5-HT, the PAR-4 agonist, or SLIGRL (Akiyama et al. 2009c).
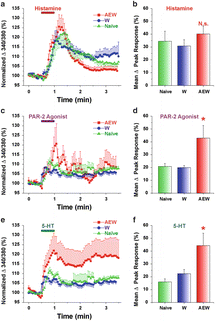
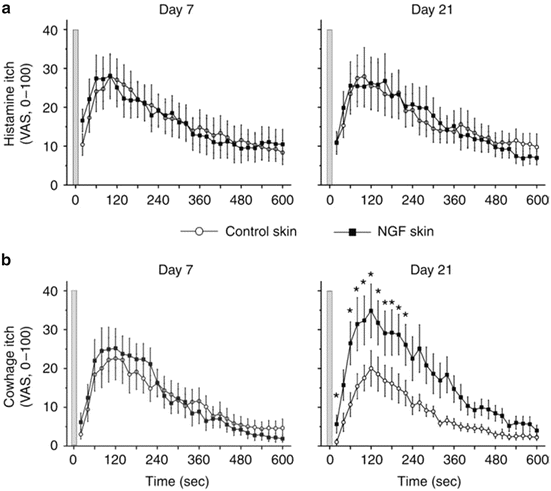

Fig. 3
DRG cells from AEW-treated mice show enhanced responses to PAR-2 agonist and 5-HT but not histamine. (a) Histamine. Mean normalized ratiometric responses of DRG cells from each treatment group vs. time relative to histamine perfusion (red bar). Error bars: SEM. (b) Mean peak response (% change from baseline) of DRG cells to histamine for each treatment group. N.s.: no significant difference compared to W. (c, e) as in (a) for PAR-2 agonist and 5-HT, respectively. (d, f) as in (b) for PAR-2 agonist and 5-HT, respectively. Asterisk, significantly different compared to W (p < 0.05, unpaired t-test). (n = 14–23/group). Naïve data from cervical DRG cells (n = 719) obtained from untreated mice. Adapted from Akiyama et al. (2010a)

Fig. 4
Nerve growth factor (NGF) sensitizes cowhage- but not histamine-induced itch. Itch sensation recorded upon (a) histamine iontophoresis and (b) cowhage spicule insertion at day 7 (left panel) and day 21 (right panel) after administration of 1 μg NGF (n = 12). In comparison with control skin, cowhage-induced itch was perceived significantly stronger at the NGF-treated sites at day 21 (marked by asterisks, Wilcoxon test, P < 0.05). Gray bars indicate the time point of histamine iontophoresis and gray cowhage application. Error bars indicate SEM. VAS, visual analog scale. Adapted from Rukwied et al. (2013)
Certain PARs are also involved in alloknesis, the phenomenon in which itch is induced by low-threshold mechanical stimulation of skin surrounding the site of the pruritic stimulus. Cowhage spicule-elicited itch is accompanied by alloknesis (Sikand et al. 2009), implying the involvement of PAR-2 and/or PAR-4 since they are activated by mucunain in cowhage (Reddy et al. 2008). Alloknesis is a common and often distressing symptom for many patients suffering from chronic itch. The neural mechanisms underlying alloknesis are poorly understood, partly due to a lack of animal models for alloknesis. To begin to investigate alloknesis, we recently developed an animal model (Akiyama et al. 2012a). C57BL/6 mice do not normally respond to innocuous mechanical stimulation of the rostral back. However, following intradermal injection of histamine and certain other pruritogens, low-threshold mechanical stimuli delivered to the skin around the injection site reliably elicited discrete hind limb scratch bouts directed to the stimulus. The time course of touch-evoked scratching had a slower onset and longer duration compared to the pruritogen-evoked scratching that usually ceased within 30 min. Touch-evoked scratching was observed following histamine, 5-HT, the PAR-4 agonist, and BAM8-22 but not SLIGRL or chloroquine (Fig. 5). Considering that alloknesis was evoked by the PAR-4 agonist but not SLIGRL, alloknesis evoked by cowhage is presumably mediated by PAR-4.
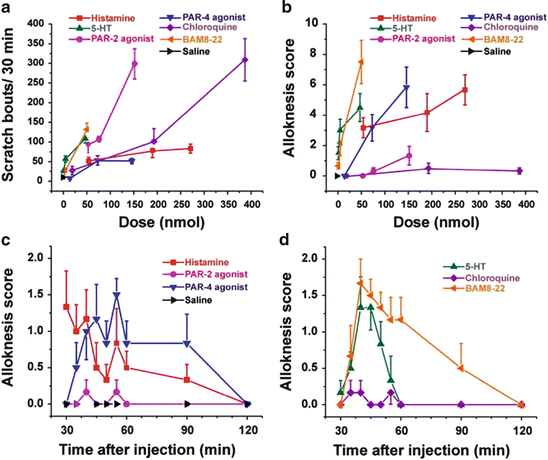

Fig. 5
Scratching and alloknesis elicited by different pruritogens. (a) Dose-response curve for scratch bouts (assessed over 30 min) elicited by intradermal injection of pruritogens indicated in each figure. Error bars: SEM (n = 6/group). (b) Dose-response curve for alloknesis score elicited by the same pruritogens. (c) Time course of alloknesis for histamine (271 nmol/10 μl), PAR-2 agonist SLIGRL-NH2 (76 nmol/10 μl), PAR-4 agonist AYPGKF-NH2 (146 nmol/10 μl), and saline vehicle. (d) Time course of alloknesis for 5-HT (47 nmol/10 μl), chloroquine (193 nmol/10 μl), and BAM8-22 (50 nmol/10 μl). Adapted from Akiyama et al. (2012a)
4 Protease-Activated Receptors and Pain
PARs have been shown to play a role in modulating nociception. PAR-2 is expressed in nociceptive primary sensory neurons, and its activation leads to hyperalgesia (Amadesi et al. 2004; Dai et al. 2004, 2007; Wang et al. 2012). PAR-2 is involved in joint, visceral, and somatic pain through the sensitization of TRPV1, TRPA1, TRPV4, and P2X3 (Amadesi et al. 2004; Dai et al. 2004, 2007; Sipe et al. 2008; Helyes et al. 2010; Lam et al. 2012; Wang et al. 2012; Poole et al. 2013). In contrast, PAR-1 and PAR-4 agonists exert antinociceptive effects such as increased thermal and mechanical nociceptive withdrawal thresholds (Asfaha et al. 2002, 2007; Auge et al. 2009; Karanjia et al. 2009; Annahazi et al. 2012) with one exception (McDougall et al. 2009). High doses of PAR agonists can induce inflammation, presumably explaining the pro-nociceptive action of PAR-4 agonists in the study of joint pain (McDougall et al. 2009).
5 Protease-Activated Receptors as Target Molecules for Future Treatments of Chronic Itch
The studies discussed above provide strong evidence for the participation of PARs in acute itch as well as itch sensitization. Thus, the clinical treatment of chronic itch is likely to benefit from the development of drugs directed at PARs. There are two strategies to block PAR-mediated itch signaling: (1) inhibit the protease agonists of PARs and (2) antagonize PARs. Nafamostat mesilate, a serine protease inhibitor, inhibited scratching evoked by tryptase and compound 48/80 as well as spontaneous scratching in NC mice exhibiting atopic dermatitis-like skin lesions (Ui et al. 2006; Tsujii et al. 2009). Leupeptin, a protease inhibitor, inhibited scratching evoked by compound 48/80 as well as passive cutaneous anaphylaxis in mice (Ui et al. 2006; Zhu et al. 2009a). The chymase inhibitor SUN13834 inhibited spontaneous scratching in a mouse dermatitis model induced by repeated treatments of hapten (Terakawa et al. 2008). Similar to the serine protease inhibitors, PAR-2 antagonists have been used successfully to inhibit itch-related behavior in mice. Scratching evoked by tryptase and compound 48/80 was inhibited by the PAR-2 antagonist FSLLRY, as well as by an anti-PAR-2 antibody (Ui et al. 2006). Spontaneous scratching in dry skin-treated mice, as well as in NC mice, was inhibited by an anti-PAR-2 antibody (Tsujii et al. 2009; Akiyama et al. 2010a). FK506 is a drug that provides temporary itch relief in atopic dermatitis. Its antipruritic effect might be due to inhibition of PAR-2-mediated signaling (Nakano et al. 2008). Overall, PARs are attractive targets for the development of treatments for itch. PAR-2 antagonists, such as the novel low molecular weight, non-peptide PAR-2 antagonist GB88, have been developed recently. Their antipruritic effects await future testing (Suen et al. 2012). Further studies will reveal the detailed mechanisms underlying the role of PARs in acute and, especially, chronic itch.
Acknowledgments
The work was supported by grants from the National Institutes of Health DE013685, AR057194, and AR063228.
References
Akiyama T, Carstens E (2013) Neural processing of itch. Neuroscience 250:697–714CrossRefPubMedCentralPubMed
Akiyama T, Carstens MI, Carstens E (2009a) Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol 102(4):2176–2183CrossRefPubMedCentralPubMed
Akiyama T, Merrill AW, Carstens MI, Carstens E (2009b) Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci 29(20):6691–6699CrossRefPubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


