Prescriptions can be presented to the pharmacy in written form or via telephone, fax, or computer, depending on individual state laws. Prescribers can include physicians, dentists, veterinarians, nurse practitioners, and physician assistants. Prescriptions are usually filled in an outpatient pharmacy for patient use on an ambulatory basis.
B. A medication order, like a prescription, is a written order for the preparation and administration of medication, issued by a licensed medical practitioner who is authorized to prescribe. Medication orders are intended for patients in an inpatient (e.g., institutional, hospital) setting.
II. Understanding Prescriptions and Medication Orders
A. Prescriptions should contain the following information:
- Patient information
a. The patient’s name, age, address, and telephone number.
- Date
a. The date the prescriber wrote the prescription order.
- Name of the product
a. The drug name can be written as either the generic or trade name.
- Strength of the product
a. The strength should always be included to avoid misinterpretation. Exceptions include if only one strength is commercially available, or if otherwise inappropriate (e.g., devices). Strength may also be excluded in products that consist of a combination of two or more drugs for which only one drug:drug concentration ratio is commercially available.
- Dosage form
a. If only one form is commercially available, the dosage form may not be included. Chapter 1 contains more information on product strength, dose, and dosage form, as well as a list of common dosage forms.
- Quantity of medication to be dispensed
a. The quantity represents the number of units or dosage forms (e.g., tablets, ounces, grams) to be dispensed. This amount may not be included in the prescription if the dispensing quantity can be calculated from the physician’s directions and duration of therapy (e.g., 3-month supply). Quantity may also be written as q.s. (a sufficient quantity) or q.s. ad (a sufficient quantity to make).
- Directions for preparation
a. Instructions to the pharmacist for compounding or preparing a product may be included.
- Directions for labeling
a. Prescriber’s instructions to the pharmacist on what information should be included on the prescription label.
- Directions for the patient on the prescription label
a. Instructions for the patient on how to use the medication properly. Tables 6-1 and 6-2 list terminology and abbreviations frequently used for prescription directions.
(1) Route of administration. Chapter 1 contains information on drug administration routes.
(2) Dosage and dosage schedule. The quantity of medication to be taken by the patient and the schedule (frequency and/or time) of administration.
- Refill information
a. The prescriber’s instructions for the number of refills that may be dispensed from the prescription order. If no information is provided, no refills are authorized. A prescriber may also write “no refills” or “NR” on the prescription order.
- Prescriber information
a. Prescriber’s name, address, and telephone number.
b. Prescriber’s Drug Enforcement Administration (DEA) number. Chapter 7 outlines the formula for validating DEA numbers.
(1) The DEA number is a unique number assigned to practitioners, hospitals, and pharmacies to keep track of controlled drug distribution and prescriptions.
c. Prescriber’s signature (unless the prescription is received by telephone).
TABLE 6-1. Common Abbreviations Used in Prescriptions and Medical Orders
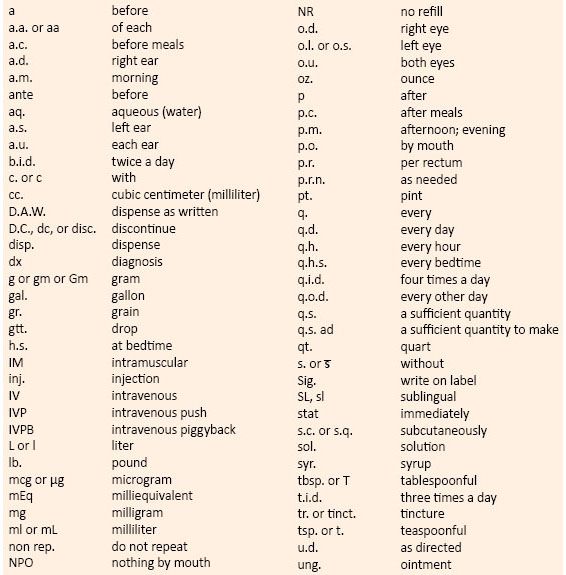
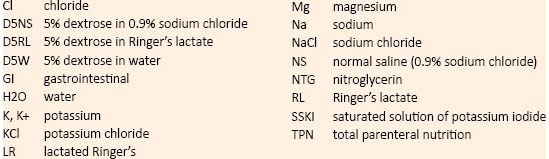
TABLE 6-2. Common Abbreviations Related to Drug Products or Diseases
B. Medication orders
- Patient information
a. While every medication order may not include in-depth patient information (diagnosis, concurrent therapies, etc.), the patient profile should contain this information as transcribed from previous orders. Medication orders typically include the following information:
(1) Patient’s name, birth date, room number, identification number
(2) Indication for use of the medication (why the drug is being ordered)
(3) Allergies or other information necessary to process the medication order
- Date the medication order was written
- Time of day that the medication order was written
a. Time is an important factor in the institutional setting where patients are cared for on a 24-hour basis.
- Name of the product
a. Generic and/or trade name of the product
- Strength of the product
a. The strength should always be included to avoid misinterpretation. Exceptions include if only one strength is commercially available, or if otherwise inappropriate (e.g., devices).
Strength may also be excluded in products that consist of a combination of two or more drugs for which only one drug:drug concentration ratio is commercially available.
- Dosage form
a. The dosage form may not be included if only one form is commercially available. Chapter 1 contains more information on product strength, dose, and dosage form, as well as a list of common dosage forms.
- Prescriber information
a. Name and signature of the prescriber. The prescriber is usually the patient’s primary physician but may be another attending physician or resident. The signature of the prescriber is not required when the order is received verbally, either by telephone or through another authorized health care professional. In these cases, the name or initial of the recipient (e.g., attending registered nurse or pharmacist) must be included on the medication order.
- Directions for preparation
a. Instructions to the pharmacist for compounding or preparing a product may be included.
- Directions for administration
a. Instructions for the nurse or other health care provider on how to administer the medication properly.
(1) Route of administration. Chapter 1 contains information on drug administration routes.< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue



