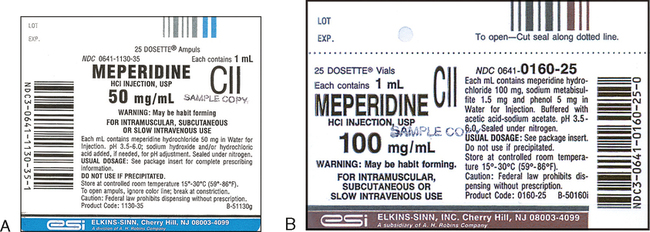
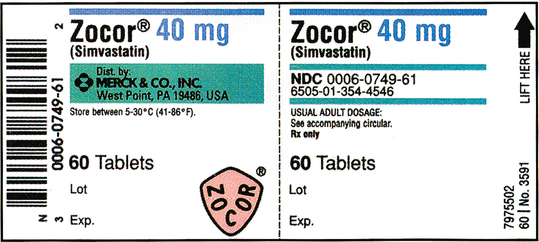
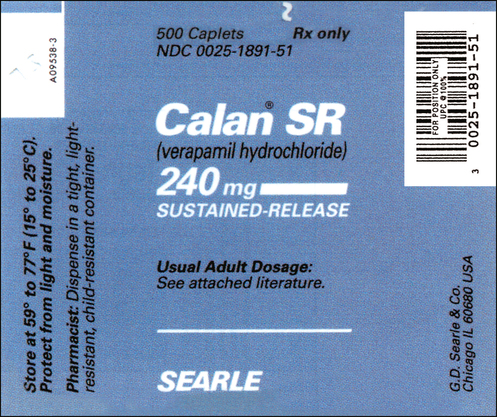
CHAPTER 13 After reviewing this chapter, you should be able to identify: 1. The trade and generic names of medications 2. The dosage strength of medications 3. The form in which a medication is supplied 4. The total volume of a medication container where indicated 5. Directions for mixing or preparing a drug where necessary The nurse should be able to recognize the following information on a medication label. Examples include morphine, phenobarbital, and atropine (Figure 13-1). Another example of a medication commonly used in the clinical setting and often seen with only the generic name on the label is Demerol. Demerol is the trade or brand name; however, it is often seen with the generic name only (meperidine). Figure 13-2 shows Demerol labels with various strengths. Note that only meperidine is indicated on the label. As stated previously, by law, the generic name must be identified on all medication labels. Notice that on all labels shown in Figures 13-1 and 13-2 the acronym USP appears after the name of the medication. USP is the acronym for United States Pharmacopoeia, which is one of the two official national listings of medications. The other is the National Formulary, NF. You will see these initials on medication labels. They are placed after the generic name. Do not confuse these abbreviations with other initials that designate a special form of a medication, such as CR, which means controlled release. The trade name is also referred to as the brand name or proprietary name; it is the manufacturer’s name for the medication. Notice that the brand name is very prominent on the label and is capitalized. It is important to remember that different manufacturers may market a medication under different trade names. The trade name is followed by an ®, which is the registration symbol. Some medications may have the abbreviation™ after the trade name, which stands for trademark. See Figures 13-3 and 13-4 for the labels for Zyvox oral suspension and Glyset, both manufactured by Pharmacia & Upjohn Company. Figure 13-5 shows the label for Zocor. Zocor is a trade name identified by the ® registration symbol. The name underneath in smaller print, Simvastatin, is the generic or official name of the medication. The dosage strength of the Zyvox shown in Figure 13-3 is 100 mg (the weight and specific unit of measurement) per 5 mL. The dosage strength of the Zocor tablets shown in Figure 13-5 is 40 mg per tablet (the weight and specific unit of measurement). Some medications, such as the label for Veetids shown in Figure 13-6, may have two different but equivalent dosage strengths shown on the label. Veetids has a dosage strength of 125 mg (per 5 mL) or 200,000 units (per 5 mL). The prescriber therefore could order the medication in either unit of measurement. Dosage strength can be expressed in different systems of measure. Some labels may state the dosage strength in apothecary and metric measures (e.g., nitroglycerin). Some oral liquids may state household measures (e.g., each 15 mL [one tablespoon] contains 80 mg). Sometimes you may see solutions expressed (dosage) as a ratio or percentage. Refer to the labels in Figures 13-7 and 13-8; notice that dosage strength is also expressed in milligrams per milliliters. The form specifies the type of preparation available in the package. • Examples of forms include tablets, capsules, liquids, suppositories, and ointments. Solutions may be indicated by milliliters (mL) and described as oral suspension or aqueous solution. Some medications are available in powder or granular form or as patches. • Labels may also indicate abbreviations or words that describe the form of the medication. Examples include CR (controlled release), LA (long acting), DS (double strength), SR (sustained release), and XL (long acting). • Abbreviations that describe the form of a medication indicate whether the medication has been prepared in a way that allows extended action, or slow release, of the active ingredient. Often, these medications are given less frequently. Examples are Procardia XL, Inderal LA, and verapamil SR. See Figure 13-9 of Calan SR label (verapamil hydrochloride). • Notice that bar-code symbols appear on some medication labels as thin and heavy lines arranged in a group. Bar codes are particularly important at institutions where bar coding is used as part of the medication distribution system. Refer to the bar codes indicated on the labels in Figures 13-4 to 13-6 and 13-8 to 13-11. Bar codes can also be used for stock reorder. The route of administration describes how the medication is to be administered. In Figure 13-5 (Zocor tablets), the total amount of tablets in the container is 60 tablets, whereas the dosage strength is 40 mg per tablet. In Figure 13-9 (Calan SR caplets) the total amount of caplets in the container is 500 caplets, whereas the dosage strength is 240 mg per caplet. On the Zyvox label, shown in Figure 13-3, 150 mL is the total volume when reconstituted (mixed) and the dosage strength is 100 mg per 5 mL. 100 mL is the total volume of the Amoxil once reconstituted (mixed), but the solution or liquid contains 125 mg per 5 mL (Figure 13-12). When medication comes in a powdered form, the directions for how to mix or reconstitute it and with what solution are found on the label or package insert. The directions for reconstitution should be followed exactly as stated on the label for accuracy in administration. See the directions on the label in Figure 13-11 for Nebcin; notice that directions are indicated for mixing or reconstituting under Preparation of Solution. In Figure 13-12 (Amoxil label) the directions for mixing Amoxil are shown on the side of the label under Directions for mixing. Reconstitution is discussed in Chapter 19. Medications may come with warnings, alerts, or precautions that are related to safety, effectiveness, or administration and need to be followed. Storage alerts might also be on the label. These precautions, warnings, and alerts may be printed on the label by the manufacturer or added by the pharmacy that dispenses the medication. Always read precautions or special alerts carefully, and follow the instructions given precisely. Examples of precautions, warnings, or alerts on a label may include the following: shake well, protect from light, may be habit forming. Example: Refer to the lower left side of the Amoxil label in Figure 13-12 (Keep tightly closed. Shake well before using.). Medication labels contain information such as the expiration date (which may be indicated with the abbreviation Exp). Expiration dates indicate the last date on which a medication should be used. Typically, the date appears as the month/year. This information can be found on the back or side of a label. Medications requiring reconstitution provide specific expiration instructions. Refer to the Amoxil label in Figure 13-12 (“Discard suspension after 14 days.”). In the hospital setting medications that have expired should be returned to the pharmacy. Note the expiration date on the furosemide label (8/2012) in Figure 13-13. Note: Expiration dates must always be checked on medications, and expiration dates are always present on actual prescriptions. The labels shown in this text may not all have expiration dates because they are used solely for educational purposes. Other information on a medication label includes the following: Storage Directions—This section of the medication label provides information as to how a medication should be stored to prevent the medication from losing its potency or effectiveness. Usually information is given on the label relating to temperature for storing the medication. Refer to meperidine label 100 mg per mL (see Figure 13-2, B), and Zocor label (see Figure 13-5). When medications come in a powdered form and must be reconstituted, storage information is usually indicated on the label telling how long the medication is effective once it has been reconstituted. Refer to Nebcin label (see Figure 13-11) and Amoxil label (see Figure 13-12). Lot/Control Numbers—Federal law requires that all medication packages be identified by a lot/control number. This number is important in the event that medications have to be recalled. Refer to furosemide label (see Figure 13-13), lot number 401803C. Abbreviations such as USP (United States Pharmacopoeia), NF (National Formulary)—USP and NF are the two official national lists of approved medications. Special guidelines are given to the manufacturer related to use and placement of these initials on medication labels. On the Adrenalin label in Figure 13-7 notice that USP follows Epinephrine Injection. Notice the placement of USP in the lidocaine label (see Figure 13-8). Some medication labels may indicate the usual medication dosage on the label, or the label may say to read the package insert for complete information. The usual dosage tells how much medication is given in a single dose or in a 24-hour period. See Figure 13-2, B (meperidine), which refers to the package insert, and Figure 13-12 (Amoxil), which states the usual adult and child dose. Medications considered controlled substances are classified into schedules that rank them according to their abuse potential and physical and psychological dependence. They are ranked from Schedule I to Schedule V. Medications that have the highest abuse potential are Schedule I, and medications with the lowest or limited abuse potential are Schedule V medications. Refer to the labels for meperidine in Figure 13-2, which state that they are in Schedule II (notice the “C” with II). The label for Sinemet, which is the trade name for an antiparkinsonian drug, indicates that the medication contains carbidopa and levodopa. The first number specifies the amount of carbidopa, and the second number represents the amount of levodopa. This is further indicated on the bottom of the label. See sample labels in Figures 13-14 and 13-15. Septra, an antibacterial that is also manufactured under the trade name Bactrim, is a combination of trimethoprim and sulfamethoxazole. For example, a Septra tablet contains 80 mg of trimethoprim and 400 mg of sulfamethoxazole. Septra DS contains 160 mg of trimethoprim and 800 mg of sulfamethoxazole. See the labels for Septra in Figures 13-16 and 13-17.
Reading Medication Labels
READING MEDICATION LABEL
Generic Name

Trade Name


Dosage Strength
Example:


Form


Route of Administration
Total Amount in Container
Examples:

Directions for Mixing or Reconstituting a Medication
Precautions
Expiration Date

Additional Information
Controlled Medication Labeling
Medication Labels for Combined Drugs
Example 1:

Example 2:


![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree