(1)
Department of Pathology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Keywords
Congenital and acquired thymic cystsDevelopmental cystsThymic hyperplasiaMediastinitisEctopic thyroid and parathyroidThymolipomaCastleman’s diseaseA variety of reactive, developmental, inflammatory, and tumorlike conditions can occur in the mediastinum (Table 1.1). Congenital and developmental cysts can present as a mass lesion in the mediastinum and may occur in a variety of settings. Congenital cysts usually occur in younger patients and are unilocular and small; they can arise anywhere along the anatomic course of embryonic descent of the thymus, including the neck. Developmental cysts can arise from displaced or ectopic remnants, such as foregut cysts and enteric duplication cysts. Acquired cysts can arise as a result of underlying inflammatory processes and can grow to be quite large and multiloculated. Thymic hyperplasia is another reactive process that presents as enlargement of the thymus and clinically can simulate a malignancy. Inflammatory conditions affecting the mediastinum include acute and chronic inflammation (mediastinitis), granulomatous processes related to sarcoidosis (affecting primarily mediastinal lymph nodes) or fungal infection, and end-stage fibrosing inflammation resulting in idiopathic sclerosing mediastinitis. Other developmental abnormalities that occur with some frequency in the mediastinum are the presence of ectopic thyroid or parathyroid tissue, which may give rise to tumor growths secondary to hyperplasia, benign nodules, or development of malignancy. Finally, a variety of benign tumorlike conditions in this anatomic compartment can lead to tumor masses that may be confused with malignancy, including thymolipoma, thymofibrolipoma, and Castleman’s disease.
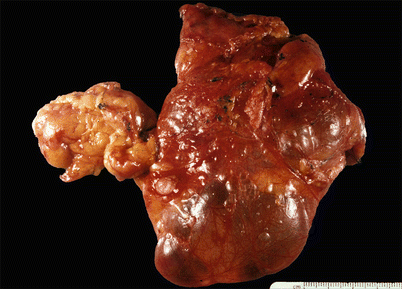
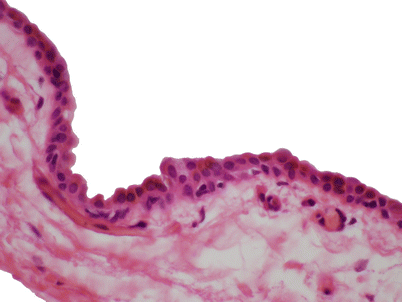
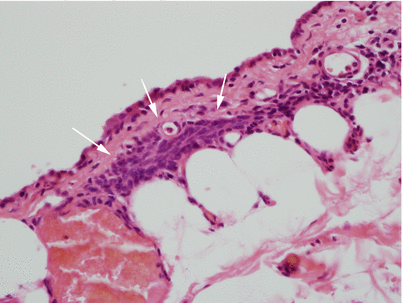
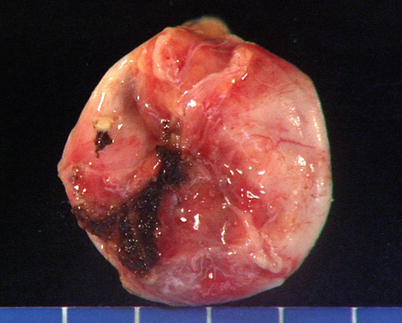
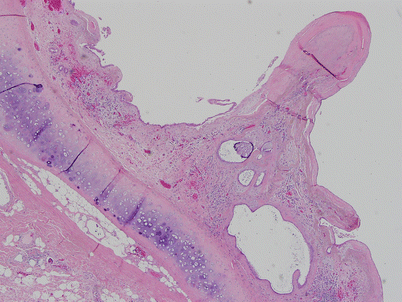
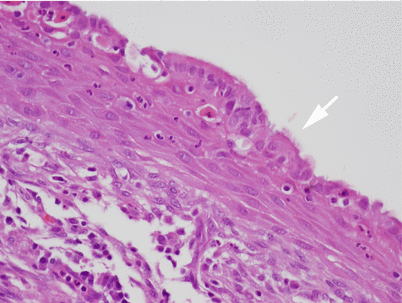
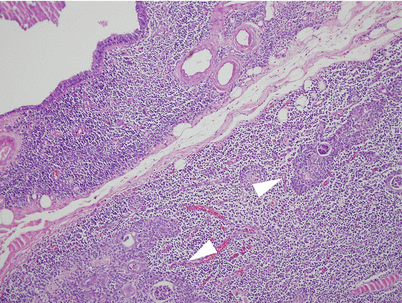
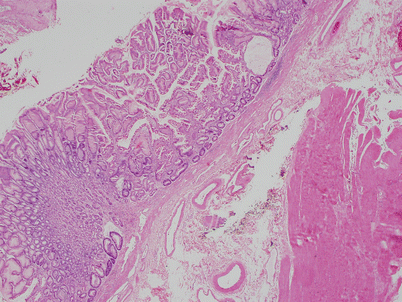
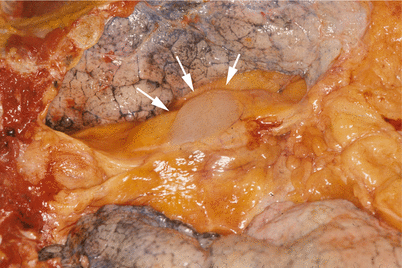
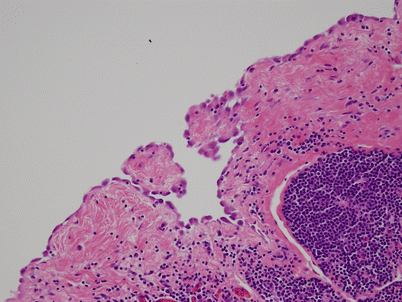
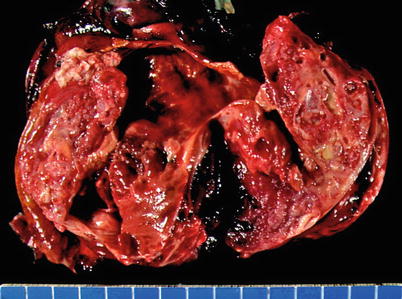
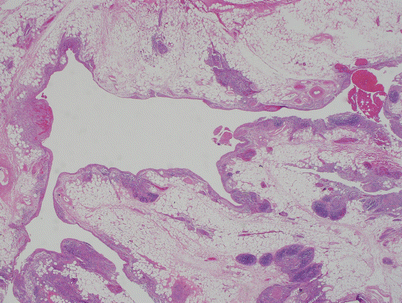
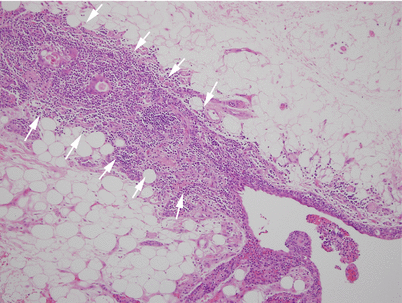
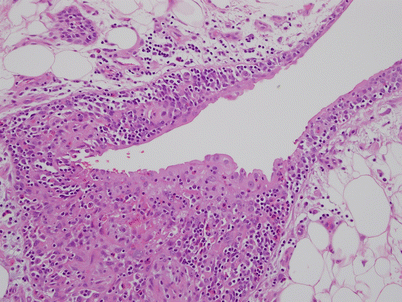
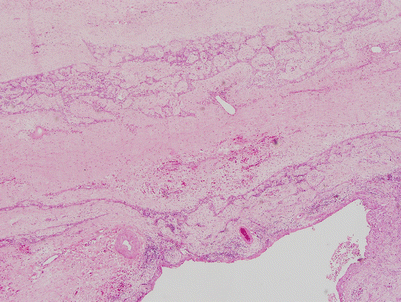
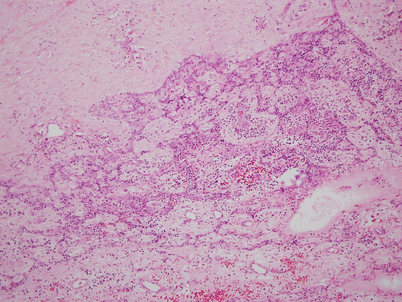
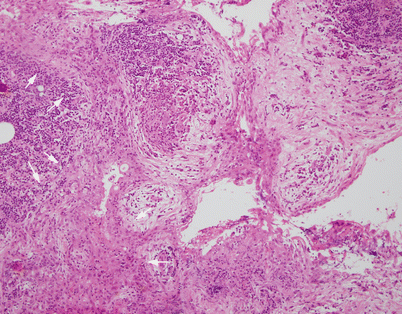
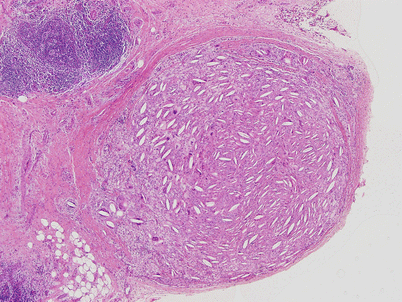
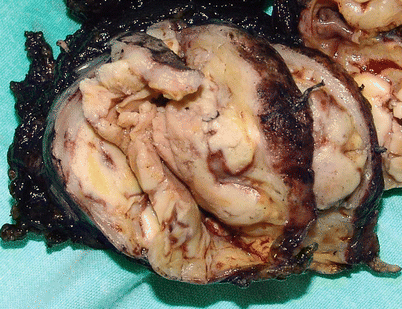
Table 1.1
Reactive, developmental, inflammatory, and tumorlike conditions of the mediastinum
Process | Condition |
|---|---|
Reactive and developmental conditions | Congenital thymic cysts |
Foregut cysts (bronchogenic, enteric) | |
Pericardial (mesothelial) cysts | |
Acquired multilocular thymic cysts | |
Thymic lymphoid hyperplasia in myasthenia gravis and other autoimmune disorders | |
“Pure” thymic hyperplasia | |
Physiologic thymic involution of the adult | |
Inflammatory conditions | Acute and chronic mediastinitis |
Granulomatous mediastinitis | |
Idiopathic sclerosing mediastinitis | |
Ectopic growths | Ectopic thyroid tissue (multinodular goiter, adenomas, carcinomas) |
Ectopic parathyroid tissue (hyperplasia, adenoma, carcinoma) | |
Tumorlike conditions | Thymolipoma |
Thymofibrolipoma | |
Castleman’s disease |

Fig. 1.1
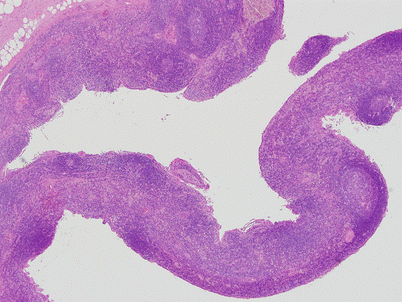
Congenital thymic cyst shows a distended, unilocular cavity lined by thin translucent walls and filled with fluid. Notice the portion of normal thymus attached at the end of the cyst. This tumor developed in a 15-year-old boy who presented with stridor and shortness of breath owing to the large size of this particular cyst (approximately 10 cm in greatest diameter)

Fig. 1.2
Histologic appearance of a congenital thymic cyst, showing a single layer of flattened cuboidal epithelium. The lining in congenital thymic cysts can also contain stratified squamous epithelium and sometimes columnar ciliated epithelium. The contents of the cyst usually consist of clear, serous fluid. Note the absence of inflammation in the wall of the cyst

Fig. 1.3
Another area in the wall of a congenital thymic cyst. Notice a small island of involuting thymic remnants beneath the lining in the wall of the cyst (arrows). This thymic remnant is composed of bland-appearing spindle cells with dispersed nuclear chromatin and scant cytoplasm reminiscent of the involuting thymus. Thymic remnants are rarely identified in the walls of congenital thymic cysts

Fig. 1.4
Foregut cyst of the mediastinum. This 4-cm cyst was incidentally found in a 23-year-old woman during a routine chest X-ray and showed a thickened, fibrous wall with a smooth and shiny outer surface. It was located at the bifurcation of the trachea and main bronchi and was easily detached and removed by blunt dissection. Foregut cysts most commonly arise in the middle or posterior mediastinum. They can occasionally communicate with the trachea or main bronchi and are more common in young adults and children, although older individuals also may be affected

Fig. 1.5
Histologic examination of a foregut cyst shows a lining composed of columnar ciliated epithelium, with hyaline cartilage, smooth muscle, and mucus glands in the walls of the cyst. Infection is a common complication and may lead to lung abscess formation in cases associated with a tracheobronchial fistula

Fig. 1.6
At higher magnification, a portion of a foregut cyst shows immature (spindled), stratified squamous epithelium with well-developed intercellular bridges showing partial maturation of the luminal surface toward ciliated columnar epithelium (arrow). The identification of cartilage in the wall of the cyst is the most reliable way to identify cysts that originate from the bronchi. Otherwise, the term “foregut cyst” would be an appropriate designation

Fig. 1.7
Foregut cyst showing a lining composed of columnar ciliated epithelium in close proximity with clusters of residual involuting thymic epithelium in the wall of the cyst (arrowheads). The thymic epithelium in such instances can undergo hyperplastic changes, not to be confused with the development of thymoma

Fig. 1.8
Example of a foregut cyst of an enteric type presenting as a mass in the posterior mediastinum in a 15-year-old boy with dysphagia. The lining of the cyst is composed of gastric-type epithelium with a readily identifiable muscularis propria. Occasionally, ciliated columnar epithelium can be identified in these cysts when they arise from the esophagus. Another term used for these cysts is “enteric duplication cyst.” The lining can be of either a gastric or enteric type. Rarely, some foregut cysts show admixtures of gastric, enteric, and bronchial epithelium

Fig. 1.9
Mesothelial (pericardial) cyst incidentally found at autopsy (arrows). The cyst shows a smooth, translucent wall bulging from the pericardium. The cyst was filled with clear, serous fluid. Similar cysts can occur higher in the anterior mediastinum and arise from the pleural reflection; these are designated as “pleural mesothelial cysts”

Fig. 1.10
Histologic appearance of a mesothelial cyst of the anterior pericardium shows a single layer of round to polygonal mesothelial cells. Rarely, the mesothelium can show foci of papillary mesothelial hyperplasia, not to be confused with malignant mesothelioma

Fig. 1.11
Cut surface of an acquired multilocular thymic cyst in a 56-year-old woman, showing multiple distended cystic cavities filled with hemorrhagic fluid. The walls of the cyst are thickened and edematous and show pinpoint foci of hemorrhage and cholesterol granulomas. These cysts can grow to very large proportions and become symptomatic. Extensive sampling is required to rule out the possibility of cystic degeneration of an underlying malignant neoplasm

Fig. 1.12
Histologic appearance of multilocular thymic cyst, showing dilated cystic cavity surrounded by dense lymphoid aggregates. The lining of the cyst is made up of simple cuboidal epithelium to stratified squamous epithelium. Focal areas of hemorrhage and inflammation are commonly present in the wall of the cysts

Fig. 1.13
Acquired multilocular thymic cyst at higher magnification, showing the lining of the cyst in continuity with residual thymic epithelium inside the walls of the cyst (arrows). The residual thymic epithelium is accompanied by a small lymphocytic component and can be seen to be in continuity with dilated Hassall’s corpuscles

Fig. 1.14
Detail of the lining in an acquired multilocular thymic cyst, showing the cyst cavity lined by simple cuboidal epithelium originating from a remnant of thymic tissue in the wall of the cyst. Notice the admixture of the epithelium with small T lymphocytes

Fig. 1.15
More advanced stage in a multilocular thymic cyst, showing dense fibrosis of the walls of the cyst secondary to chronic inflammation and netlike branching of hyperplastic thymic epithelium surrounded by the fibrous tissue

Fig. 1.16
Higher detail from an area of fibrosis in a multilocular thymic cyst, showing complex branching of thymic epithelium displaying a sieve-like architecture, with thin, elongated strands of thymic epithelial cells circumscribing dense areas of collagen in a fibroepitheliomatous fashion. This appearance is very distinctive in long-standing multilocular thymic cysts with prominent fibrotic changes in the walls

Fig. 1.17
Acquired multilocular thymic cyst showing severe inflammation of the walls with pseudoepitheliomatous hyperplasia. Notice the tongues and strands of squamous epithelium arising from the luminal surface of the cyst and infiltrating into the wall of the cyst (arrows). These reactive changes can sometimes be quite prominent and display mild cytologic atypia and even mitotic figures, simulating an invasive squamous cell carcinoma arising from the wall of the cyst

Fig. 1.18
Cholesterol cleft granuloma in the wall of an acquired multilocular thymic cyst. In addition to hemorrhage, fibrosis, and acute and chronic inflammation, cholesterol cleft granulomas are a prominent feature often encountered in thymic cysts. Notice the admixture of foamy macrophages and multinucleated giant cells with the cholesterol clefts

Fig. 1.19
Acquired multilocular thymic cyst of lymphoepithelial type shows thickened, fleshy, and edematous walls with a fish-flesh appearance due to dense lymphoid infiltrates. Such cysts are very similar to lymphoepithelial cysts of the pancreas or salivary glands and are a common feature in children with AIDS, but they can also be seen in adult patients who are not immunosuppressed