Figure 9.1
Effect of myocardial ischemia on late Na current (lNa) and activation of the vicious circle of events due to lNa. Ranolazine inhibits Calcium overload and thereby improves myocardial ischemia
Preclinical Evidence with Ranolazine
The effect of ranolazine on sodium and calcium homeostasis and contractile function has been demonstrated in experimental models of rat hearts in which sodium overloading was caused by increased Ina [17]. Ranolazine has been shown to improve diastolic ventricular relaxation during ischaemia/reperfusion in rabbits [18], in the isolated rat heart in presence of ischaemic metabolites [19], and in ventricular myocytes of dogs with ischaemic left ventricular dysfunction. All these effects have been associated with an amelioration of myocardial ischemia in the models used.
Clinical Use of Ranolazine
Ranolazine was approved for clinical use in the USA in 2006, as add-on therapy for the treatment of chronic angina, and received a first-line indication in November 2008. The maximum dosage approved in the USA is 1 g twice daily. In Europe, ranolazine (prolonged-release tablets) was approved by the European Medicine Agency in July 2008 as add-on therapy for the symptomatic treatment of patients with stable angina pectoris who are inadequately controlled or intolerant to first-line antianginal therapies (such as beta blockers and/or calcium antagonists). The recommended initial dose in adults is 375 mg twice daily that it can be further up-titrated to the recommended maximum dose of 750 mg twice daily.
Clinical Development Program
The clinical development program of ranolazine includes earlier trials conducted with the immediate-release formulation and later clinical trials that used the sustained-release formulation. The main dose-response study for the demonstration of the efficacy of ranolazine was the Monotherapy Assessment of Ranolazine In Stable Angina (MARISA) trial [20], which randomised 191 patients to either ranolazine or placebo. The CARISA study (Combination Assessment of Ranolazine In Stable Angina) [21], assessed the effect of ranolazine 750 mg twice daily or 1 g bd as add on to atenolol, amlodipine or diltiazem in 823 patients with chronic stable angina. In the Efficacy of Ranolazine In Chronic Angina (ERICA) trial, 565 patients were randomised to receive ranolazine 1 g (bd) or placebo in addition to amlodipine 10 mg (od) for 6 weeks [22].
The Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes (MERLIN)-TIMI 36 trial, randomised 6,560 patients to receive ranolazine (initially intravenously and then 1 g bd orally) or matching placebo within 48 h from the onset of ischaemic symptoms. Patients were followed for a median of 348 days. This study was conducted in patients with acute coronary syndromes and for this reasons will not be reported in this chapter [23].
MARISA Study
This was the main dose-response study. The objectives of the trial were to assess the tolerability of three doses of ranolazine sustained-release compared to placebo and their effects on treadmill exercise performance. It was a double-blind, randomised, placebo-controlled, cross-over study enrolling 191 patients treated with 500, 1,000 and 1,500 mg ranolazine against placebo. Treatment duration was 1 week at each dose level. Improvement of exercise duration was statistically significant compared to placebo for all three doses of ranolazine from 24 s at 500 mg bid. to 46 s at 1,500 mg bid, and showing a clear dose-response pattern. However, the disproportionate increase in adverse events with the 1,500 mg bid dose led to the conclusion that doses above 1,000 mg bid should not be used.
TERISA Study
The TERISA study (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina) assessed the efficacy of ranolazine versus placebo on weekly angina frequency and sublingual nitroglycerin use in 949 diabetic patients with coronary artery disease and chronic stable angina despite treatment with up to two anti-anginal agents [24]. Patients were randomized to a parallel 8 week double-blind study of ranolazine (target dose 1 g bid) or placebo. Weekly angina episodes were significantly reduced by ranolazine compared to placebo (3.8 vs. 4.3 episodes, p = 0.008), as was the weekly use of sublingual nitrates (1.7 vs. 2.1, p = 0.003). The study showed that among symptomatic patients with diabetes and despite treatment with up to two anti-anginal agents, ranolazine was effective in reducing the occurrence of angina attacks and the use of sublingual nitrates.
CARISA Study
In this study 823 patients were randomised to either ranolazine 750 mg bid. (n = 279), ranolazine 1 g bid or placebo as add-on treatment to atenolol 50 mg od, amlodipine 5 mg od, or diltiazem 180 mg od. All patients had had chronic stable angina for at least 3 months. The pre-qualifying exercise test had to be symptom-limited and show the usual definite signs of myocardial ischaemia during exercise (0.1 mV ST segment depression). Mean age of included patients was 64 years. Mostly Whites and only about 23 % were females. Twenty-three percent were diabetics and 29 % had a diagnosis of congestive heart failure. Over 60 % were hypertensive and 58 % had suffered a previous myocardial infarction. The objective of the study was to determine the effects of ranolazine at doses of 750 mg bid or 1 g bid compared to placebo on symptom-limited exercise duration among patients with chronic stable angina treated with commonly used anti-anginal drugs. The primary efficacy end point was the change from baseline in exercise duration at trough after 12 weeks on study drugs. At baseline there were no differences between the three treatment arms; all showed an exercise duration of approximately 7 min, since one requirement was the ability to perform an exercise test with a duration of 3–9 min (modified Bruce protocol). The mean increases in exercise duration at trough were statistically significantly greater for patients treated with either dose of ranolazine SR than with placebo. A significant improvement in the occurrence of anginal episodes and the use of sublingual nitrates was also observed with ranolazine (Fig. 9.2).
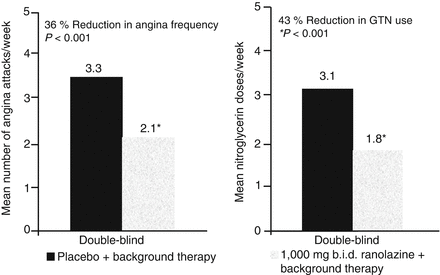

Figure 9.2
CARISA study effect of ranolazine on angina frequency and use of sublingual nitrates compared to placebo
ERICA Study
The trial assessed the effect of ranolazine sustained-release in patients with a documented history of coronary artery disease, chronic stable angina for 3 months or longer, and three or more angina episodes a week during the 2-week qualification period despite treatment with amlodipine 10 mg daily. Patients were randomised to receive either ranolazine 500 mg (bd) uptitrated to 1 g after 1 week or placebo on top of Amlodipine 10 mg. Patients were assessed at 2 and 6 weeks after initiation of the full-dose. Out of 565 randomised patients 98 % in each group completed the trial. Patients allocated to ranolazine had a significantly lower incidence of weekly episodes of angina compared with patients receiving placebo. The effect on angina was mirrored by a significant reduction in the average weekly rate of NTG consumption in patients receiving ranolazine.
Meta-Analysis on Clinical Efficacy
A meta-analysis from our group assessed the effects of ranolazine on anginal symptoms, sublingual nitrate use, functional capacity, electrocardiographic signs of myocardial ischaemia and haemodynamic parameters in patients with chronic ischaemic heart disease [25]. We analysed randomized trials assessing the effects of ranolazine compared to control regarding exercise duration, time to onset of angina, time to 1 mm ST-segment depression, weekly sublingual nitrate use and weekly angina frequency. Of 358 articles identified in the initial search, 28 were retrieved for more detailed evaluation. Thereafter, 22 studies were excluded (i.e. articles reporting data already included in previous reports) and six trials were selected for the analysis.
Compared to placebo Ranolazine treatment was found to significantly improve exercise duration by >31.8 s, time to onset of angina by 37.976 s, and time to 1 mm ST-segment depression by 36.0 s. Ranolazine treatment was found to significantly reduce weekly angina frequency compared to placebo and weekly use of sublingual nitrates. Furthermore, ranolazine treatment significantly reduced HbA1c levels by 0.429 % compared to placebo, in diabetic patients. The results of this meta-analysis have confirmed the anti-anginal and anti-ischaemic properties of ranolazine and have provided an estimate of the magnitude of its effects in clinical practice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


