Klaus Mönkemüller
DEFINITION
■ Barrett’s esophagus (BE) is an acquired condition due to a change in the normal esophageal squamous epithelium to columnar epithelium containing goblet cells. This metaplastic change can progress to low-grade dysplasia (LGD) and high-grade dysplasia (HGD), with the latter having a 5% to 10% risk of developing into esophageal adenocarcinoma.1,2
■ Radiofrequency ablation (RFA) is a safe and effective endoscopic treatment modality for BE whereby squamous tissue replaces the ablated metaplastic or dysplastic epithelium. RFA uses a bipolar electrode array to generate thermal energy to result in tissue dissipation.3
PATIENT HISTORY AND PHYSICAL FINDINGS
■ It might be difficult to distinguish symptoms of BE from gastroesophageal reflux disease (GERD) clinically, although increased duration, severity, and early age of onset for reflux symptoms as well as obesity predispose to BE occurrence.4
■ Age older than 55 years, male gender, white ethnicity, or smokers are predisposing factors for BE development and progression to dysplasia. Genetic influences may play a role, although only a small proportion (7%) of patients with BE have a documented family history of BE or esophageal cancer.5
■ Selection of patients for endoscopic treatment of BE requires a multidisciplinary approach consisting of the endoscopist, pathologist, and the surgeon.
■ Confirmation of dysplasia (high or low grade) on biopsies or endoscopic mucosal resection (EMR) specimens is done by a dedicated gastrointestinal (GI) pathologist.6
IMAGING AND OTHER DIAGNOSTIC STUDIES
■ Detailed endoscopic examination with high-definition white light of the BE segment is essential for management.
■ The extent of the BE segment should be defined using the Prague C & M classification including the length of the circumferential segment (C) and the maximal extent of the BE segment (M)7 (FIG 1).

■ Other imaging modalities that might help in delineating BE are narrow band imaging (NBI), chromoendoscopy, autofluorescence imaging, and confocal laser endoscopy. These adjuncts aid in directed biopsies.
■ Any visible lesions in the Barrett’s segment should be described using the Paris classification.8
■ Targeted biopsies are obtained from visible abnormalities, followed by four-quadrant biopsies of every 1 to 2 cm of the BE segment (Seattle protocol) and these should be reviewed by a dedicated GI pathologist.9 Nodular lesions are best staged by an EMR as described in Chapter 11.
ENDOSCOPIC MANAGEMENT
Preoperative Planning
■ Patients are given standard esophagogastroduodenoscopy (EGD) preprocedure preparation instructions with specific attention to factors that increase risk of sedation including morbidly obese patients; anatomic variants such as short neck, cervical osteophytes, cricopharyngeal hypertrophy; and prior history of surgery involving the GI tract, radiation, or documentation of previous strictures.
■ No antibiotics are required and it is desirable to minimize or stop antiplatelet/anticoagulation prior to the procedure.
■ Equipment lists for circumferential and focal ablation can be found in Tables 1 and 2.

Positioning
■ The patient is placed in the left lateral decubitus position and prepared as for a routine upper endoscopy.
TECHNIQUES
CIRCUMFERENTIAL ABLATION
Endoscopy with Inspection and Recording the Landmarks
■ Measurements are taken to map the extent of the BE showing (1) top of intestinal metaplasia (TIM), (2) proximal contiguous area of BE (M), (3) proximal level at which the BE is circumferential, (4) and the top of the gastric folds (TGF) (FIG 2).

■ Careful inspection should be made to rule out prior ulceration, strictures, previous scarring from EMR or residual nodularity as these may compromise any balloon circumferential ablation. Dilatations should be performed prior to ablation.
■ Mucosa is washed with N-acetylcysteine to clear any excess mucus and prepare the tissue for further ablation.
■ After adequate inspection and recording landmarks, a guidewire is passed into the gastric antrum and then the endoscope removed.
Sizing
■ The sizing balloon is attached to the control unit and then calibrated externally also to rule out any leaks. It is then introduced over the guidewire into the body of the esophagus, placing it 3 cm proximal to the TIM measurement.
■ Serial measurements are taken at 1-cm intervals, starting proximally and proceeding distally by inflating the device on the pedal provided. The displayed measurements on the console are then recorded by a technician or nurse. The smallest diameter treatment balloon suggested throughout the sizing is chosen as appropriate ablation catheter.
Selecting Appropriate Ablation Device
■ After sizing, the catheter is then removed, keeping the guidewire in place.
■ The smallest diameter treatment balloon suggested throughout the sizing is chosen as appropriate ablation catheter and attached to the generator.
First Ablation Pass
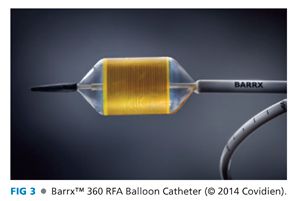
■ The Barrx™ 360 RFA Balloon Catheter (Covidien, Mansfield, MA) (FIG 3), consisting of a 3-cm electrode array encircling a 4-cm long balloon, is then passed over the guidewire into the esophagus (FIG 4A). The endoscope is intubated alongside the catheter to visualize the proximal end of the balloon, which is then positioned 1 cm proximal to the TIM.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


