Radical Nephrectomy for Renal Cell Cancer
Elias S. Hyams
Mohamad E. Allaf
Introduction
Renal cell carcinoma (RCC) constitutes ∼3% of adult malignancies and 90% to 95% of renal neoplasms. Rising utilization of abdominal imaging in the past 20 years has increased the rate of incidental diagnosis to >50%. Concurrently, there has been stage migration with a rising frequency of small tumors and localized disease at presentation. As such, nephron-sparing surgery (NSS) has been increasingly performed with the goal of preserving normal renal parenchyma and residual renal function without conceding oncological outcomes. Though NSS has become the standard of care for treatment of most small renal masses, radical nephrectomy is still the treatment of choice for larger and/or central lesions. RCC overall remains a surgical disease given its resistance to chemotherapy and radiation, though immunotherapies have been shown to have modest survival benefit in the setting of metastatic disease.
In this chapter, we review the pathology, epidemiology, clinical presentation, and surgical management of RCC with a detailed review of the various operative approaches.
Pathology
RCCs are derived from renal tubular epithelial cells and have variable histological subtypes. Differentiating between histologies provides useful information regarding prognosis, recurrence risk, familial associations, and possibly response to systemic therapies in the metastatic setting. Clear cell or “conventional” RCC is the most common subtype (70% to 80%) and is associated with loss of chromosome 3p. These tumors have a worse prognosis compared with the other main types of RCC (i.e., papillary, chromophobe). Clear cell RCC is associated with von Hippel Lindau (VHL) disease and hereditary clear cell RCC, but more frequently occurs sporadically. Clear cell disease responds better to immunotherapies should they be necessary in the adjuvant setting; in particular, IL-2 only addresses clear cell RCC.
Papillary RCC (10% to 15%) is associated with trisomy of chromosomes 7 and 17, c-met gene mutations on chromosome 7, and loss of the Y chromosome. Papillary RCC can occur in hereditary syndromes as well (“hereditary papillary RCC”) and is frequently bilateral and multifocal (up to 40%).
Chromophobe RCC (5%) may have a more indolent course than other RCC subtypes, and frequently presents at low stage and low grade. It has a close histological resemblance to oncocytoma which is a benign renal neoplasm. There can be difficulty differentiating chromophobe RCC from oncocytoma on percutaneous biopsy.
Additional subtypes of RCC are less common. These include collecting duct carcinoma, which is rare and aggressive and frequently presents with metastasis; and renal medullary carcinoma, which is associated with sickle cell trait and is extremely aggressive with a poor prognosis. Certain histological characteristics of RCC are associated with more aggressive behavior, including sarcomatoid or spindle cell variants.
Grading of RCC is an important prognostic tool. Fuhrman nuclear grading (I–IV) is based on nuclear characteristics including size, contour, and nucleoli. Higher grade is associated with a worse prognosis.
Etiology/Genetics
Tobacco smoking is the most salient risk factor for RCC with an estimated doubling of risk of cancer. Numerous other potential environmental factors (e.g., phenacetin, heavy metals) have been investigated, but none has been definitively shown to be causative. Obesity and hypertension appear to be associated with the increased risk of RCC. Acquired cystic disease from chronic end stage renal disease (ESRD) also increases the risk of RCC; the relative risk in ESRD patients may be up to 20 times higher than the general population.
RCC occurs in both sporadic and hereditary forms. Sporadic RCC is typically unilateral and unifocal, with 2% to 4% rate of bilateral disease. Hereditary forms are more commonly bilateral, and occur within several syndromes. VHL disease is an autosomal dominant disorder associated with a deletion of chromosome 3p, the VHL tumor suppressor gene. Renal cancer develops in 30% to 55% of patients with VHL. RCC is typically multifocal and bilateral, thus there may be a greater imperative to perform NSS in this population. Major clinical manifestations of VHL include clear cell carcinoma, hemangioblastomas of the central nervous system, retinal angiomas, and pheochromocytoma. Interestingly, deletion of the VHL gene is associated with both sporadic and familial forms of clear cell RCC.
Birt–Hogg–Dube syndrome is a hereditary cutaneous syndrome associated with development of fibrofolliculomas, lung cysts, pneumothorax, as well as bilateral multifocal oncocytoma and chromophobe RCC. This syndrome results from mutation of the BHD1 gene on chromosome 17.
Hereditary papillary RCC is an autosomal dominant disorder in which patients develop bilateral, multifocal papillary RCC; the specific type of tumor is considered Type 1 papillary RCC. Mutations in the c-met proto-oncogene on chromosome 7 are thought to be etiologic.
Familial leiomyomatosis and RCC result from mutation of the fumarate hydratase gene on chromosome 1. This leads to Type 2 papillary RCC as well as cutaneous leiomyomas and uterine leiomyomas. These RCC lesions tend to be more aggressive than other forms of familial RCC.
Symptoms of RCC when present may be related to bleeding, local growth, paraneoplastic syndromes, or metastasis. The classic “triad” of flank pain, gross hematuria, and a palpable flank mass is fortunately uncommon (10%) as it generally represents advanced disease. Findings suggestive of advanced disease include significant weight loss, bone pain, clinical lymphadenopathy, and poor performance status. Patients with local invasion may have pain from invasion of the abdominal wall, nerve roots, or paraspinous muscles.
Up to one-third of patients with RCC have metastasis on initial presentation. Metastases usually involve more than one site, and occur by both hematogenous and lymphatic spread. Metastatic sites from most to least frequent include lung, bone, regional lymph nodes, liver, ipsilateral adrenal gland, contralateral kidney, and brain. Systemic abnormalities may represent metastatic disease and include elevated erythrocyte sedimentation rate, hypertension, anemia, cachexia, weight loss, fever, abnormal liver function, and hypercalcemia, polycythemia, and neuropathy.
Importantly, these abnormalities may also be stigmata of a paraneoplastic syndrome, though metastatic disease should be rigorously excluded. Paraneoplastic syndromes develop in 20% to 30% of patients with RCC and result from ectopic hormone section. Substances that can be secreted include parathyroid-like substance (causing hypercalcemia), erythropoietin (polycythemia), renin (hypertension), gonadotropins, placental lactogen, enteroglucagon, insulin-like substances, prostaglandin A, and prolactin. Syndromes associated with these hormones typically reverse upon removal of the tumor; persistence is compatible with occult metastatic disease.
“Stauffer’s syndrome” is an idiopathic reversible hepatic dysfunction that occurs in up to 20% of patients with RCC. This syndrome manifests with increased alkaline phosphatase, indirect bilirubin, and altered international normalized ratio (INR). These abnormalities will reverse postoperatively in the setting of localized disease.
Venous insufficiency and thromboembolism may be consistent with tumor thrombus extending into the inferior vena cava (IVC), and may warrant an investigation for RCC. Concerning signs include lower extremity edema, pulmonary embolism, right-sided varicocele or persistent varicocele in the supine position, and dilated superficial abdominal veins.
Diagnosis of renal neoplasms is typically made on cross-sectional abdominal imaging. The gold standard exams for evaluation of a renal mass are contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). The differential diagnosis for a renal tumor may include RCC, benign neoplasm (e.g., angiomyolipoma [AML], oncocytoma, renal adenoma), abscess, lymphoma, metastasis from a separate primary lesion, pyelonephritis, renal cyst, renal infarction, and sarcoma or Wilm’s tumor.
Renal masses may be solid, cystic, or mixed. Solid masses may represent benign or malignant disease, thus contrast imaging is needed to detect “enhancement” which is generally compatible with malignancy. An increase in Hounsfeld units of >10–15 from non-contrast to arterial phase is thought to be consistent with a malignant process. However, though ∼80% of enhancing renal masses are histologically malignant upon removal, 20% will be benign (e.g., oncocytoma, fat-poor AML). Patients should be appropriately counseled regarding this possibility.
Considerations on CT/MRI include the size and position of the tumor, the status of the adrenal glands and contralateral kidney, presence of lymphadenopathy, presence of visceral metastasis, and renal vein or IVC involvement. Nodes ≥2 cm are thought to be more likely malignant, whereas smaller nodes are typically inflammatory. Presence of tumor thrombus can be detected with high sensitivity with both multiplanar CT and MRI.
Cystic renal masses are typically categorized using the Bosniak system that applies to CT appearance. This system reflects the likelihood of malignancy, with increasing complexity (thickness/number of septations, solid and/or enhancing components) increasing the likelihood of RCC. Bosniak III–IV lesions are typically treated as carcinoma, II–IIF lesions are followed radiographically, and Bosniak I or “simple” cysts do not require follow-up. Calcifications are thought to be associated with malignancy but are not pathognomonic.
Renal ultrasound frequently detects incidental masses in the course of evaluation for flank or abdominal pain. Ultrasound may assist in characterizing a mass as solid versus cystic, and a cyst as simple versus Complex; however, ultrasound has a poor sensitivity for malignancy compared with cross-sectional imaging. Ultrasound may be used as a screening study in patients with impaired renal function who cannot receive iodinated contrast or gadolinium.
We do not routinely recommend percutaneous biopsy of renal masses as it does not affect management decisions in most cases. Though the accuracy, sensitivity, and specificity of percutaneous biopsy have improved in recent series, it still has imperfect negative predictive value reported as low as 50%. Thus, a negative result may not be trusted in a young healthy patient such that surgery will be performed regardless of biopsy result. Conversely, in an elderly, comorbid patient, a biopsy showing malignancy may not lead to surgical intervention based on concerns regarding procedural risks, and the tumor may be observed such that the biopsy was superfluous and potentially harmful. Additionally, biopsy may be prone to underestimating tumor grade. Specific settings in which biopsy may be helpful include when the patient has a second malignancy, and there is concern for renal metastasis when there is suspicion for infection (abscess/phlegmon) or if there is suspicion of lymphoma based on radiographic appearance of the mass (diffuse and infiltrating rather than encapsulated).
Staging
The TNM staging system of the American Joint Committee on Cancer is most commonly used for RCC and had been validated to correspond with cancer-specific survival. See Table 1 for details of staging. Estimated 5-year disease-specific survival rates are 95% for T1 disease, 88% for T2, 59% for T3, and 20% for T4.
Preoperative evaluation for radical nephrectomy includes a complete history and physical exam. Comorbidities that might affect patient positioning or surgical approach (e.g., severe cardiopulmonary disease, kyphoscoliosis) should be assessed. The presence of chronic kidney disease (CKD) or comorbidities affecting risk of renal dysfunction (e.g. diabetes, hypertension) should be noted and factor into decision-making regarding partial versus radical nephrectomy. Prior abdominal or retroperitoneal surgery should be considered regarding the desired surgical approach. Family history of renal masses or genetic syndromes should be elicited. Assessment of patient performance status is critical if cytoreductive nephrectomy is being considered.
A complete metabolic panel including liver function tests is sent preoperatively. Serum calcium should be assessed to screen for bone metastasis or paraneoplastic syndrome. A complete blood count should be assessed to rule out polycythemia due to erythropoietin-producing tumors or lactoferrin-producing tumors with anemia. Glomerular filtration rate (GFR) should be calculated to stratify for risk of renal dysfunction postoperatively.
Table 1 American Joint Committee on Cancer (AJCC) TNM Staging System for RCC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Operative planning based on CT or MRI is critical. The size and position of tumor are noted for radical nephrectomy to ensure Gerota’s fascia is not violated near the tumor and to anticipate areas of gross inflammation, and for partial nephrectomy to plan the depth/extent of resection. Relationships of the tumor to the collecting system, renal sinus fat, and the renal hilum should be assessed. The number and position of renal arteries and veins should be noted. Involvement of the adrenal gland and other adjacent structures should be assessed, as should presence and extent of renal vein or caval thrombus. The contralateral kidney should be examined for abnormalities.
Chest imaging is necessary preoperatively to rule out lung metastasis. A chest X-ray is an acceptable first-line test, and a chest CT can be performed if there are equivocal findings. A bone scan can be considered if there is bone pain, fracture, hypercalcemia, or elevated alkaline phosphatase. A head CT can be considered if there is an abnormal neurological exam.
Preoperative infarction via angioembolization is rarely utilized, but can be useful for large tumors in patients with arterialized caval tumor thrombus or medial extension of the tumor that may prohibit early ligation of the renal artery. Infarction can also be used for palliation in the setting of severe bleeding.
There are numerous management strategies for RCC, including radical nephrectomy (open vs. laparoscopic), partial nephrectomy (open vs. laparoscopic/robotic), ablation (percutaneous vs. laparoscopic), and active surveillance.
Radical Nephrectomy
Radical nephrectomy is indicated for large and/or complex renal tumors that are not candidates for NSS. This procedure entails removal of Gerota’s fascia and its contents including kidney and perirenal fat. The adrenal gland is also present within Gerota’s fascia; however, it is generally spared unless there is direct invasion or metastasis. Laparoscopic radical nephrectomy (LRN) is currently the standard of care based on equivalent long-term cancer control compared with open surgery, with hastened convalescence, and decreased postoperative pain. This procedure is typically performed transperitoneally but can be performed retroperitoneally as well. Hand-assisted LRN can be performed to decrease the learning curve of the procedure. There has been recent interest in further minimizing the invasiveness of nephrectomy through single-incision surgery, which has been shown to be safe and feasible in those with appropriately thin body habitus. Open radical nephrectomy (ORN) is rarely performed for localized kidney cancer, though it is the approach of choice in the presence of caval tumor thrombus because of superior exposure to the great vessels and the ability to mobilize the liver as necessary. ORN is performed if there is concern for local invasion, plan to resect visceral metastasis, or significant lymphadenopathy in the setting of cytoreduction.
We advocate sparing the ipsilateral adrenal gland during radical nephrectomy unless there is direct involvement or if there is an abnormality suggestive of metastasis. The adrenal should also be taken for upper pole lesions in which there is concern regarding surgical margins.
We do not routinely perform a regional lymphadenectomy with radical nephrectomy, as studies have shown that it does not appear to influence outcome. Indications for a nodal dissection include suspicious lymphadenopathy on preoperative imaging and/or concerning nodes detected intraoperatively.
Tumor thrombus extending into the renal vein or IVC occurs in 4% to 10% of patients. When there is isolated renal vein involvement, the vein can be controlled beyond the edge of thrombus and standard radical nephrectomy performed. However, extension into the cava requires opening the cava for extraction of thrombus. Primary repair, patch grafting, or interposition grafting can be employed in conjunction with a vascular surgeon. Extension to the diaphragm and/or chest may require mobilization of the liver, venovenobypass, and/or cardiopulmonary bypass with circulatory arrest in conjunction with a cardiothoracic surgeon. Caval thrombus is associated with high perioperative risks and modest long-term survival rates; however, long-term survival can be achieved with radical surgery in up to 50% of the patients depending on patient selection. Important predictors of survival include tumor stage, grade, lymph node status, presence of distant metastasis, and presence of systemic symptoms at diagnosis.
When RCC presents with a single site of metastasis, radical nephrectomy and metastectomy are recommended for patients with good performance status as resection may provide a cancer-specific survival advantage. Recent reports have suggested that there is a survival advantage with resection of multiple metastases as well when technically feasible. If a metachronous single metastasis occurs, resection of that metastasis is performed. Radical nephrectomy in the setting of advanced and/or metastatic disease is performed for “cytoreduction” to be followed by systemic immunotherapy in conjunction with a medical oncologist. There is thought to be an immunological benefit to removing the primary tumor; cytoreduction has been shown to confer advantages in terms of delayed progression and overall survival in selected patients with metastasic disease. Eastern Cooperative Oncology Group (ECOG) performance status 0–1 is an important selection criterion for pursuing this treatment approach. There are specific criteria described by
Motzer and colleagues that help predict survival in patients with metastatic RCC, and may help with decision-making regarding use of systemic therapy. There is active investigation of neoadjuvant immunotherapy in the setting of metastatic RCC, and recent prospective data has suggested a survival benefit of this approach in selected good-risk patients. Importantly, RCC is notoriously resistant to chemotherapy and hormonal therapy. Radiation therapy may be considered for palliation of bone and brain metastasis, but is not effective for treatment of the primary tumor.
Motzer and colleagues that help predict survival in patients with metastatic RCC, and may help with decision-making regarding use of systemic therapy. There is active investigation of neoadjuvant immunotherapy in the setting of metastatic RCC, and recent prospective data has suggested a survival benefit of this approach in selected good-risk patients. Importantly, RCC is notoriously resistant to chemotherapy and hormonal therapy. Radiation therapy may be considered for palliation of bone and brain metastasis, but is not effective for treatment of the primary tumor.
Partial Nephrectomy
Partial nephrectomy is currently the standard of care for treatment of most small renal masses. Historically, partial nephrectomy has been used primarily for patients with imperative indications for renal preservation (e.g., solitary kidney, bilateral renal tumors, preexisting CKD, genetic tumor syndromes). However, it is presently recommended for all patients with T1a and T1b disease, when technically feasible, based on equivalent long-term oncological outcomes of radical nephrectomy and decreased long-term risk of CKD. Indeed, there has been growing evidence of the relationship between radical nephrectomy and CKD, as well as the link between CKD and risk of cardiovascular morbidity and mortality. There has been ongoing concern in the urological field that partial nephrectomy has been underutilized because of technical demands and lack of education regarding risks of CKD.
The desired approach to partial nephrectomy depends on surgeon experience, but in general a minimally invasive approach including laparoscopic and/or robotic surgery is preferred. Laparoscopic partial nephrectomy (LPN) by experienced surgeons has been shown to have long-term oncological outcomes equivalent to open surgery, with decreased perioperative morbidity and hastened convalescence. Robotic techniques have recently been applied to minimally invasive partial nephrectomy (MIPN) and have demonstrated promising initial results; improved dexterity, magnification, and surgeon ergonomics have applied naturally to tumor resection and renal reconstruction. Ultimately, however, nephron-preservation should be prioritized over a minimally invasive approach, given the long-term benefits of renal preservation which outweigh the short-term and cosmetic benefits of laparoscopy and robotics.
Partial nephrectomy, whether open or minimally invasive, generally requires temporary clamping of the renal artery during resection of the tumor to minimize blood loss and to allow visual assessment of the deep tumor margin. Clamping of the renal vein may also be performed, though sometimes will cause back-bleeding and require release. Renal ischemia should be minimized to reduce the risk of acute kidney injury postoperatively and to ensure preservation of long-term renal function. Recent evidence has demonstrated that the safe duration of ischemia depends largely on non-modifiable patient factors like preexisting renal dysfunction. For open partial nephrectomy (OPN), the kidney is cooled via ice slush prior to resection to prolong the safe duration of ischemia by slowing renal metabolism; this is called “cold ischemia.” MIPN requires “warm” ischemia as there is not an opportunity to cool the kidney intracorporeally. It is typically thought that 30 minutes of warm ischemia will minimize the likelihood of long-term renal dysfunction, though shorter ischemia times are preferred. Cold ischemia is thought to prolong this safe duration for up to 4 hours.
Ablative Treatments
Ablative treatments have also been developed to treat small renal tumors in minimally invasive fashion. Cryotherapy and radiofrequency ablation have been employed both percutaneously and laparoscopically to destroy a lesion while minimizing treatment morbidity and enabling preservation of the kidney. These approaches are typically used in older, comorbid patients, as retreatment rates are high and long-term efficacy has not been demonstrated.
Active Surveillance
Active surveillance has been increasingly utilized as a management strategy for patients with small renal masses and significant comorbidities that might compromise the safety of active intervention. Many small renal masses (20% to 30%) will be benign, and additional malignant lesions will have indolent biology; in an older, sicker patient, it may be reasonable to observe a mass for growth as a proxy for its aggressiveness, with the thought that the risks of surgical intervention exceed the potential benefits. The typical threshold for “safe” observation of a renal mass is 3 cm. Most small renal neoplasms that are observed have slow growth (0.15 cm/y) and incidence of metastasis in these observed populations is quite low. Ultimately, the decision to pursue active treatment versus surveillance requires a detailed conversation with the patient regarding the risks and benefits of alternative management strategies.
Incisions
Selection of the appropriate incision is critical to enable optimal exposure during open renal surgery. Factors influencing the type of incision include size and location of the tumor, body habitus of the patient, prior abdominal or retroperitoneal surgery, adjacent organ involvement, and/or the need for bilateral renal operations. The type of incision can globally be divided into the flank approach and the anterior approach (see Fig. 1).
Flank/Thoracoabdominal Approach
We favor the flank approach for open surgery as it enables an extraperitoneal dissection that avoids the abdominal viscera. The flank approach may be contraindicated in patients with severe cardiopulmonary disease or scoliosis based on positioning concerns. An incision is made from the interspace between the 10th and 11th ribs, and directed toward an area ∼1 cm above the umbilicus (see Fig. 2). Incisions below the 11th rib may not enable adequate access to the renal hilum, adrenal gland, and upper pole of the kidney. A traditional flank incision involves positioning the patient perpendicular to the bed. The patient can also be positioned in a modified flank position to improve access to the central abdomen, or depending on surgeon preference. This entails placing the patient with the ipsilateral abdomen tilted up 45 degrees. The patient is carefully strapped to the table and the table can be tilted in either direction as needed.
A thoracoabdominal incision is utilized for large upper pole masses or management of caval tumor thrombus on the right side. This incision may start as high as the eighth to ninth interspace and is directed toward the umbilicus. This incision enables excellent exposure of the upper pole and adrenal gland, as well as the suprarenal IVC. Frequently, the pleural space will be entered, which can be expeditiously closed at the end of the case with evacuation of pleural air.
For a flank incision, the patient should be positioned with the tip of the 12th rib over the kidney rest. An “axillary roll” is placed under the upper chest, though not in the axilla per se, to avoid direct compression of the axillary nerve and vessels. The legs are positioned with flexion of the lower leg to 90 degrees and straight extension of the upper leg, with careful padding. The contralateral arm can be tucked at the patient’s side, or extended perpendicular to the body on an arm board. The ipsilateral arm can be positioned in padded fashion over the patient’s chest above the xiphoid, or extended over
the bed and secured within a padded armrest. The table is generally flexed and kidney rest is utilized to increase the space between the costal margin and iliac crest.
the bed and secured within a padded armrest. The table is generally flexed and kidney rest is utilized to increase the space between the costal margin and iliac crest.
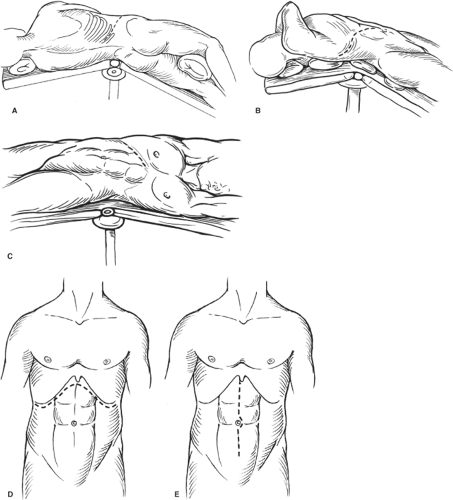 Fig. 1. Types of incisions used in nephrectomy. A: Full flank (thoracoabdominal). B: Anterior thoracoabdominal. C: Subcostal. D: Chevron. E: Midline. |
A flank incision is typically made starting in the 10th to 11th rib interspace and extending toward the supraumbilical abdomen to the edge of the rectus muscle. Incision of skin and subcutaneous tissue is performed through the latissimus dorsi and external oblique fascia and muscle, continuing through the internal oblique and transversus abdominus. Transversalis fascia is divided near the tip of the 11th rib with care to avoid entering the pleural cavity, if possible. The retroperitoneal space is then developed by blunt and sharp technique. Resection of the rib is rarely needed to obtain the desired exposure. Care is made not to injure the intercostal neurovascular bundle which runs typically along the inferior border of the rib.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


