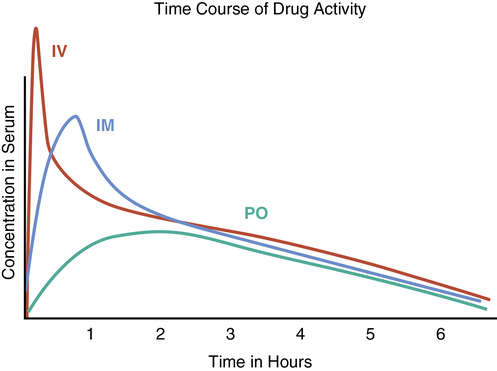
1. Identify training and competency requirements for personnel who are compounding sterile preparations. 2. Discuss causes of medications errors and the technician’s role in a prevention plan. 3. Define quality assurance, and list the major components of a quality assurance program. 4. Describe the compounded sterile preparation risk levels, including examples, quality assurance, and media fill test procedures. Any direct or physical contact with critical site areas poses the greatest threat of risk to the patient, and compounding personnel must always be conscientious of this fact. A technician must have a responsible attitude when performing aseptic compounding. Some errors may occur that would not be recognized by a pharmacist checking a final IV, such as the occurrence of a touch contamination (without being seen by the pharmacist), a wrong strength of an ingredient, or a syringe pulled back to the correct amount but not actually added to the admixture (Figure 11-1). Preparation usually occurs in the International Organization for Standardization (ISO) Class 5 area with minimal supervision of a pharmacist who is often staged in the order entry area or ante area. Final check of a retail prescription can easily include verification of the amount of tablets found in the bottle. How does a pharmacist measure the amount of additive in the admixture respectively? • Recheck calculations and interpretations of orders. This may be accomplished by simply allowing another technician or pharmacist to read the same order. • Reconfirm confusing or specialty orders. This may be done by reviewing websites (such as the Institute for Safe Medical Practices (ISMP) at http://ismp.org/) for high-risk drug information and sound-alike/look-alike drugs, or printed pharmacy references, such as Drug Facts and Comparisons or Mosby’s Drug Guide for Nursing Students. • Always check reference materials for unusual doses, and consult the pharmacist—especially for TPN or chemotherapy orders—before preparing the compounded product. • Check for drug incompatibilities. Use reference sources, such as the Handbook on Injectable Drugs by Trissel or 2013 Intravenous Medications by Gahart and Nazareno, to verify proper diluents, stability, and any special storage or preparation information. • Do not store sound-alike drugs or look-alike drugs on the same shelf. Follow standards of practice and procedures to verify these drugs, and ask someone to check with you. For a complete listing of sound-alike/look-alike drugs, see the ISMP website (http://ismp.org/). • Always work as a team to ensure the best patient care and continue to gain knowledge in the field. The IV technician is part of a team that should continuously look for ways to improve and expand knowledge about the quality of sterile preparations. • Always continue to gain knowledge about new medications through continuing education and by staying current in the practice to ensure that the most up-to-date information and practices are used. • Right patient: Be certain that names, birth dates, and any other identifying information specific to the patient is checked when interpreting the order and preparing the IV therapy. • Right medication: Check the order for accuracy and completeness. Use references, such as Trissel’s Handbook on Injectable Drugs,5 and package inserts for dilution, storage, and mixing instructions. • Right strength: Check the order for the correct dosage or strength, and label it accurately. • Right route: Check references for the appropriate route, such as IV push, piggyback, or intramuscular (IM) injection. • Right time: Verify the directions for the proper intervals for infusion, for labeling, and for the storage requirements.
Quality assurance and medication error prevention
Introduction
Medication errors
Patient rights of administration
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Quality assurance and medication error prevention