FIGURE 42-1 A 2-D CT scan image showing complete occlusion of the left inferior pulmonary veins (LIPVs) and severe stenosis of both left and right superior pulmonary veins (RSPVs).
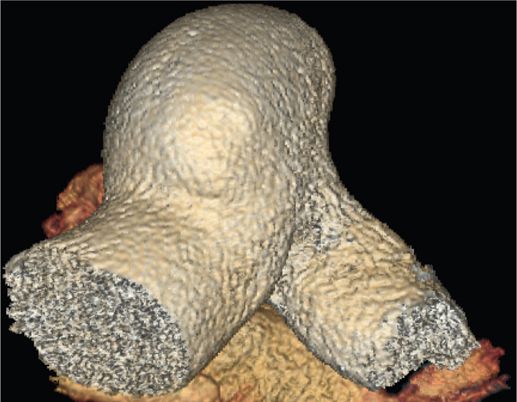
FIGURE 42-2 A 3-D CT scan image of the same patient (Figure 42-1) showing an enlarged pulmonary artery due to severe stenosis in three out of the four pulmonary veins.
EPIDEMIOLOGY
Pulmonary vein stenosis/occlusion is defined as >70% narrowing of a PV and affects 3.4% of patients following catheter ablation of atrial fibrillation. The incidence of PV stenosis is partly explained by the method of ablation and partly by the experience of the operator and the volume of cases performed.1–3 The incidence rate of PV stenosis is decreasing due to advances in intraprocedural imaging modalities and in improved ablation methods that limit the burning in the antrum of the PV,2 but given the increasing and widespread use of catheter ablation as a treatment modality for atrial fibrillation, the overall incidence of this complication may rise.
ETIOLOGY AND PATHOPHYSIOLOGY
Pulmonary vein stenosis is a clinical condition caused by delivery of radiofrequency energy within or at the orifice of the PVs. The exact etiology and pathophysiology of PV stenosis after catheter ablation of atrial fibrillation is not completely understood. Application of radiofrequency lesions within the PVs or close to the ostium of PVs is the culprit. Animal models of PV stenosis suggest periadventitial inflammation and collagen deposition as the likely mechanisms leading to stenosis formation.4 This is supported by imaging findings of fibrosis in perihilar PV tissues and presence of inflammatory protein precursors in involved PV areas.5–7 Extensive ablation of PVs in dogs was shown to result in necrotic atrial myocardium interspersed with macrophages and red cells after 2 weeks and replacement of necrotic myocardium by collagen and appearance of organized thrombus by 4 weeks. Occlusion of PVs accompanied by cartilaginous metaplasia happens around 6 to 8 weeks, followed by replacement of necrotic atrial muscle with collagenous matrix and neovascularization in about 10 to 14 weeks after ablation.6 The series of steps that lead from the application of radiofrequency energy around or inside the PV ostia to the stenosis of PVs can be summarized as:
• Metaplasia
• Proliferation of the elastic lamina/intima
• Hyperplasia
• Neovascularization
• Fibrosis and endovascular contraction
• Thrombosis
Clinical presentation of PV stenosis is widely variable in patients.1–4 Severe stenosis of a single PV can be asymptomatic in a large number of cases. In symptomatic patients, the clinical presentation of PV stenosis can include any of the following symptoms:
• Chest pain
• Dyspnea
• Decreased exercise tolerance
• Cough
• Hemoptysis
• Fever
• Recurrent lung infection
• Pulmonary hypertension (is rare and requires severe stenosis of multiple PVs)
The severity of clinical symptoms is related to the number of involved PVs, the severity and length of stenosis, and the time course of stenosis formation.4,8
DIAGNOSIS
The diagnosis of PV stenosis after catheter ablation of atrial fibrillation relies on a high index of suspicion and obtaining appropriate imaging studies. Misdiagnosis is very common in patients with PV stenosis (pulmonary embolism, lung cancer, pneumonia, and new onset of asthma are most commonly misdiagnosed) because symptoms may occur far from the procedural time.1–3 This is why timely imaging following catheter ablation is crucial even in asymptomatic patients. Indeed, most of the cases with PV stenosis are asymptomatic and may progress insidiously. Those who are symptomatic demonstrate a myriad of nonspecific symptoms.
Chest x-ray is usually not helpful in diagnosing PV stenosis. As demonstrated in the case history above, transesophageal echocardiogram (TEE) does not always provide clear images of the PVs in order to rule out PV stenosis. CT scan with contrast and MRI with contrast are the main imaging modalities used in diagnosis of PV stenosis9,10 (Figure 42-3). Many groups recommend assessment of PV diameter using CT scan or MRI 3 months after PVI together with comparison of preablation measurements to ensure best identification of PV stenosis after ablation because the caliber remains relatively stable beyond 3 months after an ablation11 (Figure 42-4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree