Pulmonary Risk and Ventilatory Support
Jay A. Johannigman
Bryce R.H. Robinson
Eric W. Mueller
Richard D. Branson
Introduction
The pulmonary system includes a complex milieu, which includes the conducting airways, pulmonary parenchyma, the alveolar capillary interface, an intricate mechanism for control of breathing, complex muscular interplay, and elaborate defense mechanisms. Yet, despite the sophistication of the respiratory system, pulmonary complications are the single most common source of morbidity in the critically ill surgical patient. Postoperative pulmonary complications include atelectasis, bronchospasm, bronchitis, pneumonia, exacerbation of chronic lung disease, and pulmonary embolism (PE) (Table 1). Uniformly, these complications result in a predictable set of symptoms including tachypnea, hypoxemia, and hypercapnia followed by respiratory failure each of which are associated with increased morbidity and mortality. Arozullah and colleagues found the need for reintubation and/or mechanical ventilation occurred in only 3% of patients in the VA database of almost 100,000 postoperative patients. Importantly, mortality in this group increased from 1% in those without respiratory failure to 27% in those with respiratory failure. Development of postoperative respiratory failure requiring mechanical ventilation results in an increased length of stay from 4.5 to 28 days. The definition of a postoperative pulmonary complication is often elusive. Radiographic atelectasis following cardiothoracic surgery is common, but rarely results in pulmonary symptoms or morbidity. This results in both overreporting and underreporting of pulmonary complications. The literature reveals an incidence of pulmonary complications from 2% to 19% after general surgical procedures and 8% to 39% following cardiothoracic surgery.
Postoperative pulmonary complications occur most commonly in those undergoing major abdominal and/or thoracic procedures. Other risk factors include advanced age, history of chronic obstructive pulmonary disease (COPD), history of obstructive sleep apnea (OSA), use of nasogastric tubes, malnutrition, renal failure, and duration of anesthesia. Diaphragmatic dysfunction, ventilation-perfusion (V/Q) mismatching, and a reduction in functional residual capacity (FRC) routinely occur after general anesthesia and surgery. Paralysis during surgery results in a cephalic movement of the diaphragm in the dorsal lung as a consequence of the weight of the abdominal contents. Controlled positive-pressure ventilation results in preferential ventilation of nondependent (ventral) lung units. As pulmonary blood flow is gravity dependent, intraoperative ventilation exacerbates V/Q inequalities and promotes dorsal atelectasis. General anesthesia has also been shown to inhibit intrinsic pulmonary defense mechanisms including altered alveolar macrophage function, disruption of the mucociliary escalator, and diminished surfactant release. Severe chronic lung disease continues to be a barrier to more complex open thoracoabdominal procedures. The evolution of minimally invasive surgery has facilitated postoperative pulmonary function and recovery in such patients. Pain control including epidural catheter analgesics has also reduced morbidity in patients with major thoracic injury. Comparisons of laparoscopic to open procedures demonstrate that in some cases (cholecystectomy and esophageal cancer) pulmonary complications are reduced, while in other procedures pulmonary complications are unchanged. This chapter includes sections on pulmonary anatomy and physiology, risk assessment, and management of acute lung injury (ALI) including mechanical ventilation, prevention and treatment of ventilator-associated pneumonia (VAP), and adjunctive surgical measures to treat complex intrathoracic infections.
Table 1 Common Postoperative Pulmonary Complications and Risk Factors | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The lungs lie lateral to the mediastinum in the thoracic cavity. The lung surface is covered by a single cell layer of pleura, which also lines the parietal surface. A minute amount of pleural fluid is present to lubricate the lung/thorax interface reducing friction. The pleural space under normal circumstances is not a space at all, but can markedly increase in disease states. The right lung has three lobes and the left has two, including the lingula, and each lobe contains two to five segments with tertiary bronchi, which can be visualized by bronchoscopy. The basic functioning unit is the alveolus. The terminal alveolar unit is a
grape-like cluster of individual air sacs often sharing a common wall surrounded by a web of capillaries, giving the lung its elastic nature. Ventilation-perfusion in the lungs based on an upright model divides the lung into three zones. In zone I (the upper lung fields), alveolar pressure is greater than both arterial and venous blood pressure resulting in a high V/Q ratio. When ventilation is greater than perfusion, dead space results. In zone II (mid lung zones), arterial pressure is greater than alveolar pressure, which is greater than venous pressure and V/Q is well matched. In zone III (lower lung fields), the vascular pressures exceed alveolar pressure and V/Q is low. When perfusion exceeds ventilation, shunt results. The typical intensive care unit (ICU) patient nursed supine causes the zones to shift horizontally and V/Q dependent on gravity. In this case, the lungs are considered to be nondependent (ventral) or dependent (dorsal). Ventilation perfusion matching during ALI helps to explain the effects of positive end-expiratory pressure (PEEP) and changes in position (prone) on arterial oxygenation. Lymph drainage from the lungs occurs through the hilar and mediastinal lymph nodes to the thoracic duct via the mediastinal lymphatics.
grape-like cluster of individual air sacs often sharing a common wall surrounded by a web of capillaries, giving the lung its elastic nature. Ventilation-perfusion in the lungs based on an upright model divides the lung into three zones. In zone I (the upper lung fields), alveolar pressure is greater than both arterial and venous blood pressure resulting in a high V/Q ratio. When ventilation is greater than perfusion, dead space results. In zone II (mid lung zones), arterial pressure is greater than alveolar pressure, which is greater than venous pressure and V/Q is well matched. In zone III (lower lung fields), the vascular pressures exceed alveolar pressure and V/Q is low. When perfusion exceeds ventilation, shunt results. The typical intensive care unit (ICU) patient nursed supine causes the zones to shift horizontally and V/Q dependent on gravity. In this case, the lungs are considered to be nondependent (ventral) or dependent (dorsal). Ventilation perfusion matching during ALI helps to explain the effects of positive end-expiratory pressure (PEEP) and changes in position (prone) on arterial oxygenation. Lymph drainage from the lungs occurs through the hilar and mediastinal lymph nodes to the thoracic duct via the mediastinal lymphatics.
The critical function of the lung is gas exchange, specifically oxygen extraction from the atmosphere and transfer to the intravascular space, and elimination of carbon dioxide. The latter is virtually dependent on minute ventilation (respiratory rate), while oxygen transport is dependent on the fractional inspired oxygen concentration (FIO2), transport across the alveolar-capillary membrane, and V/Q matching. Blood oxygen content is determined primarily by hemoglobin concentration and percent oxygen saturation, while oxygen dissolved in plasma contributes minutely. Oxygen tension in the alveolus is described by the following alveolar air equation:
where PB is the barometric pressure, PH2O is the partial pressure of water vapor, which is constant under alveolar conditions at 47 mm Hg, PCO2 is the alveolar CO2 concentration, and RER is the respiratory exchange ratio (normally 0.8).
The arterial partial pressure of CO2 (PaCO2), readily available on routine blood gas measurement, can be substituted for PACO2. Normal PAO2 in room air is 95 to 100 mm Hg and PaO2 is 85 to 95 mm Hg. The degree of V/Q mismatching will determine the shunt fraction of unsaturated blood. Shunt fraction is normally 2% to 5%, but can be significantly increased in the face of atelectasis, pneumonia, ALI, and acute respiratory distress syndrome (ARDS). The shunt fraction can be estimated by measurement of the alveolar-arterial (A-a) O2 gradient. This is defined as:
This is normally 10 mm Hg, but markedly increases in disease, which limits gas exchange across the alveolar capillary membrane. The ratio of PaO2/FIO2 is commonly used to assess the severity of pulmonary dysfunction in disease states such as ALI, ARDS, and pneumonia in the mechanically ventilated patient. The normal value is over 500. A value <300 indicates ALI and <200 indicates ARDS, when observed concurrently with bilateral infiltrates on chest x-ray and a pulmonary capillary wedge pressure <18 mm Hg. The calculation of intrapulmonary shunt requires a pulmonary artery catheter, mixed venous blood from the pulmonary artery, and the measurement of cardiac output.
The PaCO2 normally is responsible for central respiratory drive by CO2 diffusion across the blood–brain barrier, producing a decrease in cerebrospinal fluid pH and an increase in minute ventilation. Normal PaCO2 is 35 to 45 mm Hg, but can be elevated by disorders in the control of breathing and in ALI and ARDS by dead space. Physiologic dead space is defined as the dead space to tidal volume ratio (VD/VT), which is normally 0.3 owing to the anatomical dead space. VD/VT is calculated as
where PaCO2 is used as an approximate for alveolar CO2 and PECO2 is the mixed expired CO2. In ARDS, VD/VT predicts mortality, as values >0.7 are associated with >80% mortality.
Pulmonary Mechanics and Lung Volumes
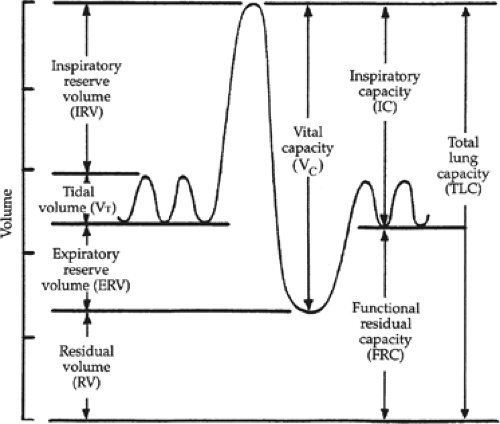
There are several lung volumes and capacities used to describe mechanical function of the lung, respiratory muscles, and chest wall (Fig. 1). Most of these are measured during pulmonary function testing. Tidal volume (VT) is the amount of air exchanged per breath during normal breathing. Vital capacity (VC) is the amount of air exchanged from peak inspiration to maximal expiration. FRC is the amount of residual air in the lung after maximal expiration. Compliance of the lung is a measure of elasticity and is defined as change in volume/change in pressure. During mechanical ventilation, dynamic compliance is measured as the difference between peak inspiratory pressure (PIP) and PEEP/VT. Static compliance is measured as the difference in plateau pressure and (Pplat) and PEEP/VT. Dynamic compliance includes the effects of airway resistance and lung compliance, while static compliance estimates alveolar pressure and lung elasticity alone. Plateau pressure is important in both lung expansion and lung injury in ARDS. Data suggest that for every increase in plateau pressure of 6 cm H2O, the development of ARDS (in patients initially without ARDS) increases by 50%. Pressure–volume
curves can be produced, which demonstrate the beginning of alveolar recruitment (lower inflection point) and alveolar overdistention (upper inflection point), and can be used to guide the choice of VT and PEEP. The pressure–volume curve is not a standard of care and should be used cautiously.
curves can be produced, which demonstrate the beginning of alveolar recruitment (lower inflection point) and alveolar overdistention (upper inflection point), and can be used to guide the choice of VT and PEEP. The pressure–volume curve is not a standard of care and should be used cautiously.
Although the ability of preoperative assessment of pulmonary function to predict the incidence of pulmonary complications postoperatively in a given patient is good, these assessments are not commonly performed. Frequent excuses for not performing preoperative testing include inadequate understanding of what test can be predicted, the absence of guidelines detailing which test should be performed and in what populations, and the utility of preventative postoperative care in all patients or just in high-risk patients.
In emergency or nonelective surgery preoperative assessment is useless as the surgical procedure must be undertaken regardless of the risk. Complete pulmonary function tests (PFTs) include lung volumes, spirometry, maximal respiratory pressures, diffusing capacities, and oximetry (Table 2). However, office-based observations such as ease of breathing, use of accessory muscles, ability to blow out a match with a wide-open mouth, and stair climbing are helpful indicators of pulmonary reserve (Table 3). Hypoxia or CO2 retention on arterial blood gas measurements is also useful in patients with known chronic pulmonary disease. Development of office-based spirometry has allowed those physicians with an interest in treating such disorders to immediate results and treatment can often be initiated early based on such testing. The objectives of PFTs are to describe dysfunction and severity, and to assess long-term prognosis as endorsed by the American Thoracic Society. The National Lung Health Program has recommended routine use of spirometry by primary care physicians.
Table 2 Pulmonary Function Tests | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 3 Preoperative Assessment of Pulmonary Risk | |||||
|---|---|---|---|---|---|
|
The primary maneuver during spirometry is the forced vital capacity (FVC) where the patient inhales to total lung capacity and then exhales out as fast and as long as possible. FVC is the difference between total lung capacity and reserve volume. The forced expiratory volume over the first second is referred to as the FEV1 and reflects degree of obstruction, usually as a consequence of smoking. The ratio of FEV1/FVC is also reported in addition to the maximal voluntary ventilation (MVV). The MVV is the maximal amount of ventilation over a 10- to 12-second period, and can vary tremendously with the fitness of the patient and the effort. Predicted values for each of these parameters can be calculated based on height, weight, and body mass index. Based on the observed values, a percent of predicted value is obtained, and is most easily interpreted. These tests can be performed before and after pharmacologic intervention such as bronchodilators, to determine the degree of reversibility of the airway disease. A significant response has been defined as either a 12% or 0.2 L or more from baseline. Tests for lung volume and diffusing capacity are more complex and not routinely done for most patients. An FEV1 <50% of predictive value, and resting hypoxemia and hypercarbia have been shown to be associated with increased postoperative pulmonary morbidity. The American Association of Anesthesiologists (ASA) has developed the ASA Class system, which can be useful in predicting intraoperative and postoperative morbidity and mortality (Table 4). This system relies more on overall physical condition of the patient, not on the results in individual measurements.
Table 4 ASA Physical Status Classification and Rate of Postoperative Complications | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The National Surgical Quality Improvement Program represents the most significant effort in identifying preoperative risk (Table 5). Factors associated with increased pulmonary morbidity include age over 60, low serum albumin, renal failure, smoking, history of COPD, high American Society of Anesthesiology (ASA) score, anesthetic time of 180 minutes or more, and type of operation. In the VA study, over 180,000 patients were studied. Postoperative respiratory failure was defined as the requirement for mechanical ventilation for more than 48 hours postoperatively, or reintubation and mechanical ventilation after postoperative extubation. This study more appropriately predicts postoperative respiratory failure, not postoperative pulmonary complications. The study did not consider other findings such as atelectasis, pneumonia, or PE in the absence of mechanical ventilation. The study included noncardiac procedures done under general or regional anesthesia. The rate of postoperative respiratory failure was just <3%. Point
values were assigned to these various factors and five classes of patients were then developed based on their scores. Class IV and V patients had predicted risks of 11% and 30%, respectively for development of postoperative respiratory failure, which closely matched the incidence from both phases of the study. Class I patients, which comprised almost half of the study population, had only a 0.5% risk of respiratory failure.
values were assigned to these various factors and five classes of patients were then developed based on their scores. Class IV and V patients had predicted risks of 11% and 30%, respectively for development of postoperative respiratory failure, which closely matched the incidence from both phases of the study. Class I patients, which comprised almost half of the study population, had only a 0.5% risk of respiratory failure.
Table 5 Veterans Affairs Pulmonary Risk Index | |||||||
|---|---|---|---|---|---|---|---|
|
Preoperative pulmonary risk has been studied thoroughly in patients who undergo thoracotomy and lung resection. Of major importance is the estimate of residual lung function to minimize the risk of permanent pulmonary dysfunction resulting in ventilator dependence. Lung cancer often occurs concurrently with COPD. Even when other risk factors are accounted for, the presence of COPD is the predominant risk factor for postoperative pulmonary complications following thoracotomy. Lung volume is actually reduced by thoracotomy alone, and typically FEV1 is reduced by 10% after lobectomy and by 30% after pneumonectomy. Methods for determining postoperative function after lung resection include PFTs, CT, and lung scanning. Split perfusion lung scanning has been found to be most accurate, as other methods consistently underestimate postoperative residual lung function. A predicted FEV1 of at least 700 mL has been used as a cutoff for resectability. With regard to prediction of postoperative morbidity and mortality, the inability to climb two flights of stairs and a percentage of predicted DLCO (derived from lung scan) <40% have consistently been shown to be associated with adverse clinical outcome.
Patients at Risk for Postoperative Pulmonary Complications
A number of chronic disease states are associated with greater risk of pulmonary complications. These include chronic respiratory disease, cardiac comorbidities, and select surgical procedures. The most significant will be reviewed here.
The Copd Patient
COPD is an independent risk factor for the development of postoperative pulmonary complications following both thoracic and nonthoracic surgery. COPD is characterized by airflow limitation and has been classified into five stages according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD). The small airways from resected lung tissue of COPD patients demonstrate inflammatory leukocytes in both the lumen and the walls. The severity of infiltration correlates with the GOLD stage, indicating the chronic inflammatory status of the lungs, which is often steroid resistant. Physiologically, COPD is characterized by decreased FEV1, alveolar hypoventilation, reduction in alveolar capillary diffusing capacity, hypoxemia, and/or hypercarbia. The GOLD guidelines state that COPD is present when the FEV1 is <80% of predicted and FEV1/FVC is <0.7.
Pulmonary Hypertension
Pulmonary hypertension (defined as a right ventricular systolic pressure of >35 mm Hg) is a significant and often overlooked preoperative risk. In patients undergoing noncardiac surgery having a New York Heart Association functional class >2, a history of pulmonary embolus, or OSA postoperative pulmonary and cardiac complications are substantially increased. Postoperative congestive heart failure, cardiac ischemic events, arrhythmias, strokes, respiratory failure, hepatic dysfunction, renal dysfunction, and the need for postoperative inotropic or vasopressor support are known postoperative complications in this group. Postoperative respiratory failure is the most common complication.
Preoperatively, risk factors in patients with pulmonary hypertension include right-axis deviation on the ECG, right ventricular hypertrophy, or a history of pulmonary embolus. When inhaled nitric oxide (iNO) is unavailable, use of intraoperative epinephrine, or having a right ventricular systolic pressure/systolic BP ratio of ≥0.66 also tends to increase perioperative morbidity and mortality.
Pulmonary hypertension patients unable to walk >332 m during a 6-minute walk test have a higher mortality rate than those who can. The presence of a pericardial effusion, the presence of septal shift, or an enlarged right atrium on echocardiogram also predicts worse outcomes. If a patient has known pulmonary hypertension, determining their preoperative response to vasodilator therapy may be useful in managing postoperative complications.
Asthma
Although some evidence suggests that patients with asthma are at greater risk for postoperative pulmonary complications, more recent studies have failed to corroborate this impression. Preoperatively, patients should continue to use their inhaled medications to optimize peak expiratory flow. Intraoperatively, tracheal intubation and dry anesthetic gases may trigger bronchospasm in these patients. Short-acting β2-agonists typically control this problem.
Smoking
A history of smoking increases the risk of pulmonary complications for patients undergoing any type of surgery. Patients who are current smokers have even greater risk.
Age
Patients >65 years of age undergoing nonthoracic surgery are at increased risk of postoperative pulmonary complications.
Obstructive Sleep Apnea
Patients undergoing surgery should be screened for OSA. Preoperative evaluation for OSA can be a simple list of questions for the patient and their bed partner as to snoring, periods of apnea, and disrupted sleep pattern. Preoperative polysomnography has not been shown to assist in preventing postoperative complications. A study of 170 patients undergoing bariatric surgery found that only 15% of patients were diagnosed with OSA, however, the actual incidence was 77%, as documented by polysomnography. In the general surgical population, the incidence of OSA has been estimated to be as low as 1% and as high as 9%. A plethora of studies have demonstrated that the presence of OSA correlates closely with increased postoperative morbidity and mortality. Sleep disturbances are exaggerated after surgery and general anesthesia. The early preoperative treatment of OSA with continuous positive airway pressure (CPAP) may reduce these risks.
Perioperative Therapies to Prevent Postoperative Complications
A number of interventions for reducing postoperative pulmonary complications have been explored (Table 6). These interventions should begin preoperatively, and continue through the intraoperative, perioperative, and postoperative periods. These
interventions should be carried out regardless of the risk of the development of PPCs.
interventions should be carried out regardless of the risk of the development of PPCs.
Table 6 Preoperative Preparation of the High-Risk Patient | ||||||
|---|---|---|---|---|---|---|
|
Smoking Cessation
Patients enrolling in a smoking cessation program 6 to 8 weeks prior to elective orthopedic surgery required less frequent postoperative mechanical ventilation. A number of other studies with varying durations of smoking cessation and operative interventions have demonstrated mixed results. It appears that in order to reduce postoperative pulmonary complications, smoking cessation must begin a minimum of 6 weeks prior to the operation.
Preoperative Corticosteroids and Bronchodilators
Preoperative treatment with a β-agonist and methylprednisolone for 5 days, may reduce the incidence of bronchospasm during intubation in patients with asthma and bronchial hyperactivity. This is more effective in patients naive to routine β-agonists than those on long-term therapy.
Anesthesia and Analgesia
Anesthetic agents may contribute to the development of PPCs by decreasing respiratory muscle tone and augmenting airway closure promoting atelectasis. Comprehensive reviews comparing the effect of general anesthesia and spinal anesthesia on postoperative complications in patients undergoing nonthoracic surgical procedures have found no difference in the rate of postoperative pneumonia. A meta-analysis evaluating the incidence of postoperative pneumonia in patients undergoing hip surgery found no differences based on anesthetic technique. Despite conventional wisdom, regional anesthesia has not been clearly established as an approach for reducing PPCs. Patients receiving pancuronium and those with residual blockade have an increased incidence of postoperative pneumonia.
Surgical Techniques
Studies examining the incidence of postoperative pulmonary complications using laparoscopic techniques compared to open techniques have generated variable outcomes and failed to favor one surgical approach over the other. However, common sense and clinical experience seems to favor laparoscopic techniques.
Lung Expansion Maneuvers
Lung expansion maneuvers have been advocated to decrease the risk of complications by counteracting the adverse effects of surgery on pulmonary mechanics, which predispose patients to atelectasis and retained secretions. Deep-breathing exercises, incentive spirometry, intermittent CPAP, and noninvasive ventilation have all been advanced as methods for lung expansion. Studies have failed to demonstrate the advantage of one technique over another and interestingly, several studies have shown that incentive spirometry has no advantage over deep-breathing exercises alone. In summary, the techniques used for lung expansion appear to be equally effective in preventing postoperative pulmonary complications. CPAP may be helpful in patients who are unable to perform deep-breathing exercises and in patients with OSA and/or obesity.
Use of Regional Anesthesia
Anesthesia is often classified into two: general anesthesia and regional anesthesia. General anesthesia refers to techniques that depress the central nervous system by a gaseous and/or intravenous delivery. Regional anesthesia refers to the delivery of pharmaceuticals directly to the spinal cord or nerves to locally anesthetize afferent and efferent neuronal pathways. Effective regional anesthesia for major thoracic, abdominal, and limb surgery often requires the injection of these drugs into the subarachnoid space (spinal anesthesia) or into the epidural space (epidural anesthesia) to create a neuraxial blockade.
The use of neuraxial blockade for major general surgical procedures is well established though the additional benefits it may confer is controversial. These benefits are thought to originate through the attenuation of the neuroendocrine stress response that is reported during surgical interventions. When compared to patients undergoing systemic analgesia, the use of regional techniques is associated with a decrease in plasma levels of cortisol, catecholamines, and proinflammatory cytokines. The reduction of spinal sympathetic stimulation in the perioperative setting has presumed advantages for coagulation, cardiovascular, pulmonary, gastrointestinal, and immunologic functions. Such techniques are appealing in that a blunted stress response during this period may translate into a reduction in morbidity and mortality, especially in patients that have additional risk due to inherent comorbidities.
When clinical outcomes are critically evaluated, the benefits of regional techniques become less clear. In a meta-analysis of 141 smaller randomized trials that included 9,559 patients, Rodgers et al. demonstrated a significant reduction in postoperative mortality for those patients that underwent neuraxial blockade. Furthermore, significant reductions in the odds of obtaining a deep vein thrombosis (DVT), PE, blood product transfusion, pneumonia, and respiratory depression were found in the blockade group. Rigg et al. examined the impact of epidural use during the operative and postoperative period in high-risk patients undergoing major abdominal or thoracic procedures when compared to a cohort receiving only systemic analgesia. This prospective, randomized trial of 915 patients demonstrated no difference in 30-day mortality. Of multiple morbid conditions that were examined postoperatively, only the rate of respiratory failure was significantly reduced in those with epidural use. In this group, there was a reduction in pain scores during the first 3 days of infusion though there was also a significant decrease in systolic blood pressure and maximal heart rate.
The implementation of such techniques in an elective surgical setting needs to be first discussed preoperatively with both the patient and in consultation with the anesthesiology team. Strong contraindications for placement include clotting defects and local sepsis at the insertion site. Clotting disorders, whether acquired or inherent, increase the risk of epidural hematoma formation. Infection at the site of placement or in the locality of insertion could lead to spinal seeding and abscess formation. Patients with poor cardiac function should be evaluated closely in light of the heightened risk of cardiac dysfunction that may occur due to the spinal sympathetic block of neuraxial local anesthetics. Such patients may benefit from only narcotic infusions or the removal of local anesthetics at the first signs of hypotension or bradycardia.
Prophylaxis for Venous Thromboembolism and Pulmonary Embolism
Venous thromboembolism (VTE), the formation of clot in the larger extremity or central veins, and PE, emboli from a large vein thrombus that occludes the pulmonary artery tree, continue to be major health issues in the United States. These clots effect
between 350,000 and 600,000 Americans annually and are directly or indirectly related to 100,000 deaths over such a period. This crisis has grown to such a magnitude that a “Call to Action” was issued by the Surgeon General of the United States in 2008. The rationale for the prevention of VTE and PE is based on the premise that almost all hospitalized patients have at least one risk factor for formation and that approximately 40% have three or more (Table 7).
between 350,000 and 600,000 Americans annually and are directly or indirectly related to 100,000 deaths over such a period. This crisis has grown to such a magnitude that a “Call to Action” was issued by the Surgeon General of the United States in 2008. The rationale for the prevention of VTE and PE is based on the premise that almost all hospitalized patients have at least one risk factor for formation and that approximately 40% have three or more (Table 7).
Table 7 Risk Factors for Development of Venous Thromboembolism | ||||||
|---|---|---|---|---|---|---|
|
Without thromboprophylaxis, the rate of VTE is 10 to 40% in medical and surgical populations (moderate risk) with a rate as high as 40% to 60% following major orthopedic surgical interventions or major traumatic injury (high risk). Vast amounts of irrefutable evidence exist stating that VTE and PE are preventable entities. Based on these works, timely evidence-based clinical practice guidelines exist for the prevention of VTE and are the basis for this brief review.
The prevention of VTE begins with the institutional-wide identification of moderate- to high-risk surgical patients. A formal, written policy for thromboprophylaxis and strategy for adherence has clear benefit. Low-risk surgical patients, those undergoing outpatient-type procedures, have no additional thromboembolic risk and likely need nothing more than early and frequent ambulation. Most general surgical procedures incur a moderate risk of VTE though a high risk is often assigned to hip or knee operations, major trauma patients, and moderate risk patients with multiple individual risk factors. Risk factors for VTE in general surgical patients accrue based on the presence of obesity, cancer, increasing age, use of general anesthesia, duration of surgery, presence of postoperative infection, and mobilization. The pathophysiologic basis of these risks is Virchow’s triad of vascular endothelial damage, venous stasis, and blood hypercoagulability. The mechanical methods of prophylaxis such as specifically graduated compression stockings, intermittent pneumatic compressions devices, and venous foot pumps have been appealing due to the lack of bleeding risk associated with such devices. Although the rate of DVT is lower with the use of these devices, no mechanical thromboprophylaxis option has been studied in such rigorous detail to impact PE or death rate, and the quality of such trials is often debated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree