Chapter 23 Psychiatry
Selective Serotonin Reuptake Inhibitors (SSRIs)
MOA (Mechanism of Action)
 The monoamine hypothesis suggests that depression is caused by a deficiency of synaptic neurotransmitters such as serotonin (5-HT), norepinephrine, and dopamine. Serotonin, in particular, is associated with mood.
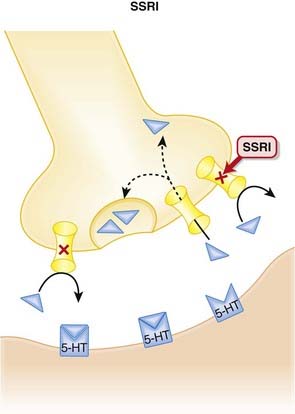
The monoamine hypothesis suggests that depression is caused by a deficiency of synaptic neurotransmitters such as serotonin (5-HT), norepinephrine, and dopamine. Serotonin, in particular, is associated with mood. Normally, 5-HT is released from presynaptic vesicles into the synaptic cleft, where it travels to postsynaptic receptors.
Normally, 5-HT is released from presynaptic vesicles into the synaptic cleft, where it travels to postsynaptic receptors. Once released from these postsynaptic receptors, 5-HT is removed from the synaptic cleft by reuptake transporters located on the presynapse. Once it is taken up presynaptically, it is degraded (Figure 23-1).
Once released from these postsynaptic receptors, 5-HT is removed from the synaptic cleft by reuptake transporters located on the presynapse. Once it is taken up presynaptically, it is degraded (Figure 23-1). SSRIs bind to this reuptake transporter, preventing the removal of 5-HT and leading to increased 5-HT available to bind to postsynaptic receptors.
SSRIs bind to this reuptake transporter, preventing the removal of 5-HT and leading to increased 5-HT available to bind to postsynaptic receptors. The clinical efficacy of antidepressants is delayed a few weeks when compared with their pharmacological actions. It is therefore hypothesized that the efficacy of these agents in the treatment of depression is related to a downstream effect.
The clinical efficacy of antidepressants is delayed a few weeks when compared with their pharmacological actions. It is therefore hypothesized that the efficacy of these agents in the treatment of depression is related to a downstream effect. There are a variety of theories as to what this downstream effect might be, although most involve either a change in receptor density or fundamental changes at the cellular level, including a reorganization of neurons.
There are a variety of theories as to what this downstream effect might be, although most involve either a change in receptor density or fundamental changes at the cellular level, including a reorganization of neurons. One of the examples of altered receptor density occurs with presynaptic 5-HT inhibitory autoreceptors. These autoreceptors reduce 5-HT release when bound by 5-HT. Excess 5-HT in the synapse because of SSRI therapy leads to down-regulation of this inhibitory autoreceptor and enhanced release of 5-HT into the synapse.
One of the examples of altered receptor density occurs with presynaptic 5-HT inhibitory autoreceptors. These autoreceptors reduce 5-HT release when bound by 5-HT. Excess 5-HT in the synapse because of SSRI therapy leads to down-regulation of this inhibitory autoreceptor and enhanced release of 5-HT into the synapse.Pharmacokinetics
 The SSRIs typically have long half-lives (24 hours or longer), allowing for once-daily administration.
The SSRIs typically have long half-lives (24 hours or longer), allowing for once-daily administration.Contraindications
 SSRIs and monoamine oxidase inhibitors (MAOIs): SSRIs increase serotonin concentrations in the synapse, whereas MAOIs inhibit the breakdown of serotonin. Concomitant use can therefore lead to excessive serotonin (see details in Side Effects). When switching from an SSRI to an MAOI, or vice versa, allow for a washout period of at least 1 to 2 weeks.
SSRIs and monoamine oxidase inhibitors (MAOIs): SSRIs increase serotonin concentrations in the synapse, whereas MAOIs inhibit the breakdown of serotonin. Concomitant use can therefore lead to excessive serotonin (see details in Side Effects). When switching from an SSRI to an MAOI, or vice versa, allow for a washout period of at least 1 to 2 weeks. SSRIs and thioridazine (an antipsychotic): Thioridazine elicits QT interval prolongation, and fluoxetine in particular enhances this effect by inhibiting the metabolism of thioridazine. A washout period of at least 5 weeks should elapse before someone who was on fluoxetine should be started on thioridazine, and fluoxetine should not be initiated for at least 2 weeks after discontinuation of thioridazine. The seriousness of these drug interactions has led to the withdrawal of thioridazine in many markets.
SSRIs and thioridazine (an antipsychotic): Thioridazine elicits QT interval prolongation, and fluoxetine in particular enhances this effect by inhibiting the metabolism of thioridazine. A washout period of at least 5 weeks should elapse before someone who was on fluoxetine should be started on thioridazine, and fluoxetine should not be initiated for at least 2 weeks after discontinuation of thioridazine. The seriousness of these drug interactions has led to the withdrawal of thioridazine in many markets.Side Effects
Serious
 Serotonin syndrome (SS) is a rare but potentially life-threatening elevation in serotonin, most commonly caused by concomitant use of SSRIs and MAOIs. Symptoms include:
Serotonin syndrome (SS) is a rare but potentially life-threatening elevation in serotonin, most commonly caused by concomitant use of SSRIs and MAOIs. Symptoms include:Non-Serious
 Sexual dysfunction may be both mechanical, as serotonin inhibits functions such as erections, ejaculation, lubrication, and orgasm, and central, as serotonin has an inhibitory effect on dopamine, a neurotransmitter believed to play an important role in arousal. Note: sexual dysfunction can also accompany depression.
Sexual dysfunction may be both mechanical, as serotonin inhibits functions such as erections, ejaculation, lubrication, and orgasm, and central, as serotonin has an inhibitory effect on dopamine, a neurotransmitter believed to play an important role in arousal. Note: sexual dysfunction can also accompany depression. Gastrointestinal (GI) distress: Serotonin receptors are also found in the gut, and serotonin appears to have an effect on GI motility (cramping, diarrhea, nausea) that becomes intolerable in some patients. Nausea and vomiting are also likely mediated by activation of serotonin receptors in the CNS.
Gastrointestinal (GI) distress: Serotonin receptors are also found in the gut, and serotonin appears to have an effect on GI motility (cramping, diarrhea, nausea) that becomes intolerable in some patients. Nausea and vomiting are also likely mediated by activation of serotonin receptors in the CNS.Important Notes
 As with other antidepressants, when an SSRI is being discontinued, the dose should be tapered gradually in order to avoid discontinuation symptoms, including dizziness, nausea, headache, and others. The incidence and severity appears to vary between SSRI, and longer half-life agents such as fluoxetine appear to be less likely to induce a discontinuation syndrome.
As with other antidepressants, when an SSRI is being discontinued, the dose should be tapered gradually in order to avoid discontinuation symptoms, including dizziness, nausea, headache, and others. The incidence and severity appears to vary between SSRI, and longer half-life agents such as fluoxetine appear to be less likely to induce a discontinuation syndrome. The use of SSRIs and other antidepressants in children is currently under review. The focus of concern is the propensity to elicit behavioral and emotional changes, including an increased risk of self-harm and suicide. All antidepressants thus carry safety warnings for use in pediatrics.
The use of SSRIs and other antidepressants in children is currently under review. The focus of concern is the propensity to elicit behavioral and emotional changes, including an increased risk of self-harm and suicide. All antidepressants thus carry safety warnings for use in pediatrics. Although they all increase serotonin levels, the SSRIs are a heterogeneous class and should not be considered interchangeable. For example, citalopram is considered to be the most serotonin-selective of the SSRIs, although it does have affinity for H1 receptors. Fluoxetine and sertraline have the highest affinity for dopamine D2 receptors, whereas paroxetine has the most potent anticholinergic effects. The clinical consequences of this pharmacologic heterogeneity have yet to be fully characterized.
Although they all increase serotonin levels, the SSRIs are a heterogeneous class and should not be considered interchangeable. For example, citalopram is considered to be the most serotonin-selective of the SSRIs, although it does have affinity for H1 receptors. Fluoxetine and sertraline have the highest affinity for dopamine D2 receptors, whereas paroxetine has the most potent anticholinergic effects. The clinical consequences of this pharmacologic heterogeneity have yet to be fully characterized. SS and neuroleptic malignant syndrome (NMS), two rare but very serious complications of SSRIs or MAOIs and antipsychotics, respectively, share some common features including fever, increased muscle tone (more hyperreflexia with SS), and autonomic instability.
SS and neuroleptic malignant syndrome (NMS), two rare but very serious complications of SSRIs or MAOIs and antipsychotics, respectively, share some common features including fever, increased muscle tone (more hyperreflexia with SS), and autonomic instability.Advanced
Drug Interactions
 The SSRIs inhibit multiple CYP450 isozymes. Fluoxetine and paroxetine inhibit the CYP2D6 isoenzyme, and this can lead to clinically important drug interactions with drugs such as tricyclic antidepressants (TCAs), carbamazepine, or vinblastine. There is also a very serious interaction with thioridazine (see Contraindications).
The SSRIs inhibit multiple CYP450 isozymes. Fluoxetine and paroxetine inhibit the CYP2D6 isoenzyme, and this can lead to clinically important drug interactions with drugs such as tricyclic antidepressants (TCAs), carbamazepine, or vinblastine. There is also a very serious interaction with thioridazine (see Contraindications).Evidence
Depression
 A 2005 Cochrane review (132 trials, N = 14,391 participants) compared fluoxetine with other antidepressants. The review found that among SSRIs, sertraline and paroxetine were more efficacious than fluoxetine in improving depression rating scores. Findings related to side effects were as follows:
A 2005 Cochrane review (132 trials, N = 14,391 participants) compared fluoxetine with other antidepressants. The review found that among SSRIs, sertraline and paroxetine were more efficacious than fluoxetine in improving depression rating scores. Findings related to side effects were as follows: The same review found fluoxetine to be less efficacious than venlafaxine or mirtazapine; fluoxetine caused less dry mouth, dizziness, sweating, and nausea compared with venlafaxine.
The same review found fluoxetine to be less efficacious than venlafaxine or mirtazapine; fluoxetine caused less dry mouth, dizziness, sweating, and nausea compared with venlafaxine.Premenstrual Syndrome
 A 2007 Cochrane review (31 trials, N = 844 participants) found that SSRIs were highly effective in the treatment of premenstrual symptoms, both physical and behavioral, compared with placebo. There were 2.5 times as many withdrawals because of adverse events among SSRI-treated subjects as among placebo-treated subjects.
A 2007 Cochrane review (31 trials, N = 844 participants) found that SSRIs were highly effective in the treatment of premenstrual symptoms, both physical and behavioral, compared with placebo. There were 2.5 times as many withdrawals because of adverse events among SSRI-treated subjects as among placebo-treated subjects.FYI
 The SSRIs were the first class of antidepressants that were discovered using “rational drug design.” The strategy was based on the observation that TCAs inhibited noradrenaline (NA) or 5-HT reuptake to various extents. Scientists then discovered some nontricyclic compounds that were also reuptake inhibitors, acting on either NA or 5-HT to varying degrees. This led to the approval of the first such agent, zimeldine, which was withdrawn from the market after a few years.
The SSRIs were the first class of antidepressants that were discovered using “rational drug design.” The strategy was based on the observation that TCAs inhibited noradrenaline (NA) or 5-HT reuptake to various extents. Scientists then discovered some nontricyclic compounds that were also reuptake inhibitors, acting on either NA or 5-HT to varying degrees. This led to the approval of the first such agent, zimeldine, which was withdrawn from the market after a few years.Tricyclic Antidepressants (TCAs)
Description
TCAs are a class of antidepressants with a common chemical (tricyclic) structure and mode of action.
MOA (Mechanism of Action)
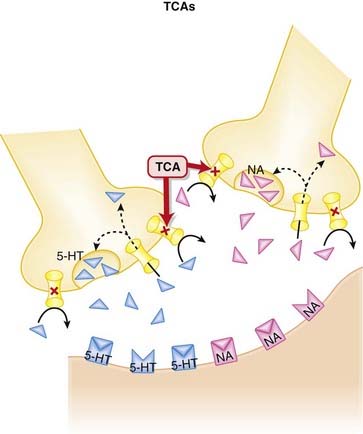
 The monoamine hypothesis suggests that depression is caused by a deficiency of synaptic neurotransmitters such as serotonin (5-HT), NA, and dopamine. Serotonin, in particular, is associated with mood.
The monoamine hypothesis suggests that depression is caused by a deficiency of synaptic neurotransmitters such as serotonin (5-HT), NA, and dopamine. Serotonin, in particular, is associated with mood. Normally, 5-HT and NA are released from presynaptic vesicles into the synaptic cleft, where they travel to postsynaptic receptors.
Normally, 5-HT and NA are released from presynaptic vesicles into the synaptic cleft, where they travel to postsynaptic receptors. Once released from these postsynaptic receptors, 5-HT and NA are removed from the synaptic cleft by reuptake transporters located on the presynapse.
Once released from these postsynaptic receptors, 5-HT and NA are removed from the synaptic cleft by reuptake transporters located on the presynapse. The TCAs inhibit the reuptake of serotonin (5-HT) and NA into the presynaptic cell body, increasing the amount of 5-HT and NA available to bind to postsynaptic receptors (Figure 23-2).
The TCAs inhibit the reuptake of serotonin (5-HT) and NA into the presynaptic cell body, increasing the amount of 5-HT and NA available to bind to postsynaptic receptors (Figure 23-2). TCAs antagonize other receptors: muscarinic, histamine (H1), adrenergic (α1) receptors. This accounts for their extensive list of side effects.
TCAs antagonize other receptors: muscarinic, histamine (H1), adrenergic (α1) receptors. This accounts for their extensive list of side effects. The clinical efficacy of antidepressants is delayed when compared with their pharmacologic actions. It is therefore hypothesized that the efficacy of these agents in the treatment of depression is related to a downstream effect.
The clinical efficacy of antidepressants is delayed when compared with their pharmacologic actions. It is therefore hypothesized that the efficacy of these agents in the treatment of depression is related to a downstream effect. A variety of theories exist regarding what this downstream effect might be, although most involve either a change in receptor density or fundamental changes at the cellular level, including a reorganization of neurons.
A variety of theories exist regarding what this downstream effect might be, although most involve either a change in receptor density or fundamental changes at the cellular level, including a reorganization of neurons.Contraindications
 Avoid concomitant use of TCAs and MAOIs: TCAs increase serotonin concentrations in the synapse, whereas MAOIs inhibit the breakdown of serotonin. Concomitant use can therefore lead to excessive serotonin. When switching from a TCA to an MAOI, or vice versa, allow for a washout period of at least 2 weeks.
Avoid concomitant use of TCAs and MAOIs: TCAs increase serotonin concentrations in the synapse, whereas MAOIs inhibit the breakdown of serotonin. Concomitant use can therefore lead to excessive serotonin. When switching from a TCA to an MAOI, or vice versa, allow for a washout period of at least 2 weeks.Side Effects
 Serious: Cardiovascular toxicities are typically only seen when high doses are administered (see “Important Notes”). In high doses TCAs impair cardiac conduction, leading to a widening of the QRS complex and heart block, often accompanied by hypotension
Serious: Cardiovascular toxicities are typically only seen when high doses are administered (see “Important Notes”). In high doses TCAs impair cardiac conduction, leading to a widening of the QRS complex and heart block, often accompanied by hypotensionImportant Notes
 The TCAs are grouped as a class based on their chemical structure. Although as a class they are considered to be 5-HT or noradrenaline reuptake inhibitors, the degree of reuptake inhibition differs markedly among agents. See Table 23-1.
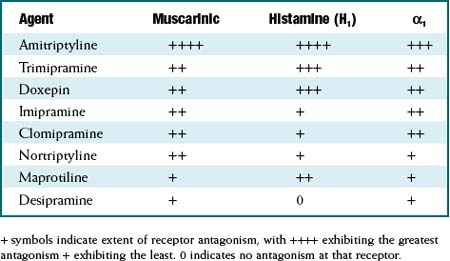
The TCAs are grouped as a class based on their chemical structure. Although as a class they are considered to be 5-HT or noradrenaline reuptake inhibitors, the degree of reuptake inhibition differs markedly among agents. See Table 23-1. Similarly, the affinities for blockade of receptors that mediate the side effects experienced by patients also differ markedly among agents. See Table 23-2.
Similarly, the affinities for blockade of receptors that mediate the side effects experienced by patients also differ markedly among agents. See Table 23-2. Because of their prominent antimuscarinic effects, TCAs should be used with caution in conditions that would be exacerbated by cholinergic antagonism: urinary retention, benign prostatic hyperplasia (BPH), glaucoma (closed angle), and increased IOP.
Because of their prominent antimuscarinic effects, TCAs should be used with caution in conditions that would be exacerbated by cholinergic antagonism: urinary retention, benign prostatic hyperplasia (BPH), glaucoma (closed angle), and increased IOP. TCAs are potentially fatal in overdose situations and have one of the highest mortality rates associated with overdose. Cardiac arrhythmias, hypotension, and central nervous system (CNS) involvement are the most common events associated with TCA overdose. TCAs undergo slow absorption; therefore a patient may arrive on his or her own at an emergency department with a fatal dose of TCAs that has not yet been absorbed. This propensity of TCAs to be fatal in overdose is particularly concerning, given the general FDA warning about increased risk of suicidality with antidepressant use.
TCAs are potentially fatal in overdose situations and have one of the highest mortality rates associated with overdose. Cardiac arrhythmias, hypotension, and central nervous system (CNS) involvement are the most common events associated with TCA overdose. TCAs undergo slow absorption; therefore a patient may arrive on his or her own at an emergency department with a fatal dose of TCAs that has not yet been absorbed. This propensity of TCAs to be fatal in overdose is particularly concerning, given the general FDA warning about increased risk of suicidality with antidepressant use.TABLE 23-1 Extent of Serotonin and Noradrenaline Reuptake Inhibition among TCAs
| Agent | Serotonin (5HT) | Noradrenaline(NA) |
|---|---|---|
| Clomipramine | +++ | + |
| Amitriptyline | ++ | ± |
| Imipramine | + | + |
| Trimipramine | 0 | + |
| Doxepin | + | ++ |
| Nortriptyline | ± | ++ |
| Desipramine | 0 | +++ |
+ symbols indicate extent of reuptake inhibition, with +++ exhibiting the greatest reuptake inhibition + exhibiting the least. 0 indicates no inhibition of the reuptake transporter for that neurotransmitter.
TABLE 23-2 Extent of Antagonism of Muscarinic, Histamine (H1), and α-1 Adrenergic Receptors among TCAs
Evidence
FYI
 TCAs have been around for 50 years. Imipramine was the first TCA synthesized, and it was based on the tricyclic structure of the antipsychotic chlorpromazine. A study in 1958 found imipramine lacked efficacy in psychosis, but, surprisingly, a subgroup of patients with depression improved on imipramine. TCAs became the drugs of choice for treating depression for the next 30 years.
TCAs have been around for 50 years. Imipramine was the first TCA synthesized, and it was based on the tricyclic structure of the antipsychotic chlorpromazine. A study in 1958 found imipramine lacked efficacy in psychosis, but, surprisingly, a subgroup of patients with depression improved on imipramine. TCAs became the drugs of choice for treating depression for the next 30 years.Serotonin Noradrenaline Reuptake Inhibitors (SNRIs)
MOA (Mechanism of Action)
 The monoamine hypothesis suggests that depression is caused by a deficiency of synaptic neurotransmitters such as serotonin (5-HT), NA, and dopamine. Serotonin, in particular, is associated with mood.
The monoamine hypothesis suggests that depression is caused by a deficiency of synaptic neurotransmitters such as serotonin (5-HT), NA, and dopamine. Serotonin, in particular, is associated with mood. Normally, 5-HT and NA are released from presynaptic vesicles into the synaptic cleft, where they travel to postsynaptic receptors.
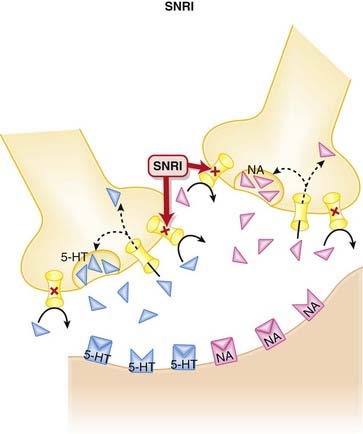
Normally, 5-HT and NA are released from presynaptic vesicles into the synaptic cleft, where they travel to postsynaptic receptors. Once released from these postsynaptic receptors, 5-HT and NA are removed from the synaptic cleft by reuptake transporters located on the presynapse. Once they are taken up presynaptically, they are degraded (Figure 23-3).
Once released from these postsynaptic receptors, 5-HT and NA are removed from the synaptic cleft by reuptake transporters located on the presynapse. Once they are taken up presynaptically, they are degraded (Figure 23-3). SNRIs bind to these reuptake transporters, preventing the removal of 5-HT and NA and leading to increased availability to bind to postsynaptic receptors.
SNRIs bind to these reuptake transporters, preventing the removal of 5-HT and NA and leading to increased availability to bind to postsynaptic receptors. Venlafaxine has much higher affinity for the serotonin reuptake transporter, and at low doses acts more like an SSRI. It is not until higher doses are used that it also blocks noradrenaline reuptake.
Venlafaxine has much higher affinity for the serotonin reuptake transporter, and at low doses acts more like an SSRI. It is not until higher doses are used that it also blocks noradrenaline reuptake. Conversely, milnacipran blocks serotonin and noradrenaline reuptake equally, whereas the other agents in this class fall somewhere between these two.
Conversely, milnacipran blocks serotonin and noradrenaline reuptake equally, whereas the other agents in this class fall somewhere between these two. The clinical efficacy of antidepressants is delayed when compared with their pharmacologic actions. It is therefore hypothesized that the efficacy of these agents in the treatment of depression is related to a downstream effect.
The clinical efficacy of antidepressants is delayed when compared with their pharmacologic actions. It is therefore hypothesized that the efficacy of these agents in the treatment of depression is related to a downstream effect. A variety of theories exist regarding what this downstream effect might be, although most involve either a change in receptor density or fundamental changes at the cellular level, including a reorganization of neurons.
A variety of theories exist regarding what this downstream effect might be, although most involve either a change in receptor density or fundamental changes at the cellular level, including a reorganization of neurons.Pharmacokinetics
 Compared with the SSRIs, the SNRIs have shorter elimination half-lives. Venlafaxine is available in an extended-release(ER) dosage form.
Compared with the SSRIs, the SNRIs have shorter elimination half-lives. Venlafaxine is available in an extended-release(ER) dosage form. Venlafaxine has an active metabolite: O-desmethylvenlafaxine. The parent and its metabolites have lower clearance in patients with hepatic cirrhosis and severe renal disease, requiring a dose reduction.
Venlafaxine has an active metabolite: O-desmethylvenlafaxine. The parent and its metabolites have lower clearance in patients with hepatic cirrhosis and severe renal disease, requiring a dose reduction. Desvenlafaxine is primarily metabolized by glucuronidation, with only minor involvement from CYP3A4.
Desvenlafaxine is primarily metabolized by glucuronidation, with only minor involvement from CYP3A4.Side Effects
 Gastrointestinal (GI) distress, attributed to inhibition of serotonin reuptake, appears to be most common with venlafaxine. Stimulation of serotonin receptors in the brain likely mediates nausea. Serotonin receptors are also found in the gut, and serotonin appears to have an effect on GI motility that becomes intolerable in some patients, leading to cramping and diarrhea.
Gastrointestinal (GI) distress, attributed to inhibition of serotonin reuptake, appears to be most common with venlafaxine. Stimulation of serotonin receptors in the brain likely mediates nausea. Serotonin receptors are also found in the gut, and serotonin appears to have an effect on GI motility that becomes intolerable in some patients, leading to cramping and diarrhea. Insomnia may occur due to stimulation of 5-HT receptors in the CNS. Both the length and quality of sleep may be impaired.
Insomnia may occur due to stimulation of 5-HT receptors in the CNS. Both the length and quality of sleep may be impaired. Sexual dysfunction, attributed to inhibition of serotonin reuptake, appears to be most common with venlafaxine. It may be both mechanical, as serotonin inhibits functions such as erections, ejaculation, lubrication, and orgasm, and central, as serotonin has an inhibitory effect on dopamine, a neurotransmitter believed to play an important role in arousal.
Sexual dysfunction, attributed to inhibition of serotonin reuptake, appears to be most common with venlafaxine. It may be both mechanical, as serotonin inhibits functions such as erections, ejaculation, lubrication, and orgasm, and central, as serotonin has an inhibitory effect on dopamine, a neurotransmitter believed to play an important role in arousal.Important Notes
 When an SNRI is being discontinued, the dose should be tapered gradually in order to avoid discontinuation symptoms, including aggression, agitation, convulsions, dysphoric mood, electric shock sensations, and others. These symptoms have been particularly evident with venlafaxine.
When an SNRI is being discontinued, the dose should be tapered gradually in order to avoid discontinuation symptoms, including aggression, agitation, convulsions, dysphoric mood, electric shock sensations, and others. These symptoms have been particularly evident with venlafaxine. The theory behind the development of the SNRIs was to try and increase the levels of two neurotransmitters (serotonin, noradrenaline) simultaneously, while avoiding many of the bothersome side effects of the TCAs, which also act as reuptake inhibitors of these two neurotransmitters. There is evidence to suggest that dual reuptake inhibition is more efficacious than selective inhibition (see Evidence section).
The theory behind the development of the SNRIs was to try and increase the levels of two neurotransmitters (serotonin, noradrenaline) simultaneously, while avoiding many of the bothersome side effects of the TCAs, which also act as reuptake inhibitors of these two neurotransmitters. There is evidence to suggest that dual reuptake inhibition is more efficacious than selective inhibition (see Evidence section). Reboxetine was the first noradrenaline selective reuptake inhibitor, approved in Europe for treatment of depression. It was rejected by the U.S. Food and Drug Administration (FDA) because of concerns over poor efficacy.
Reboxetine was the first noradrenaline selective reuptake inhibitor, approved in Europe for treatment of depression. It was rejected by the U.S. Food and Drug Administration (FDA) because of concerns over poor efficacy. All antidepressants carry an FDA warning about increased suicidality, particularly in younger (<25 years of age) patients. The mechanism has not been established and there is not enough data to determine whether a lower risk exists for some antidepressants compared with others. These concerns must also be balanced against the potential for increased risk of completed suicides in untreated depression.
All antidepressants carry an FDA warning about increased suicidality, particularly in younger (<25 years of age) patients. The mechanism has not been established and there is not enough data to determine whether a lower risk exists for some antidepressants compared with others. These concerns must also be balanced against the potential for increased risk of completed suicides in untreated depression.Evidence
Venlafaxine versus Fluoxetine for Depression
 A 2005 Cochrane review (10 trials, N = 1831 participants) compared fluoxetine with venlafaxine. The authors found venlafaxine to be significantly better than fluoxetine (an SSRI) for improving depression rating scores. Some side effects were more common with venlafaxine than with fluoxetine, including dry mouth, dizziness, sweating, and nausea.
A 2005 Cochrane review (10 trials, N = 1831 participants) compared fluoxetine with venlafaxine. The authors found venlafaxine to be significantly better than fluoxetine (an SSRI) for improving depression rating scores. Some side effects were more common with venlafaxine than with fluoxetine, including dry mouth, dizziness, sweating, and nausea.Venlafaxine for Generalized Anxiety Disorder
 A 2003 Cochrane review (8 trials, N = 2058 participants) examined the efficacy of various antidepressants for generalized anxiety disorder (GAD). Based on two trials, the authors found venlafaxine to be statistically better than placebo for treatment response, assessed by Clinical Global Impression (CGI) scores.
A 2003 Cochrane review (8 trials, N = 2058 participants) examined the efficacy of various antidepressants for generalized anxiety disorder (GAD). Based on two trials, the authors found venlafaxine to be statistically better than placebo for treatment response, assessed by Clinical Global Impression (CGI) scores.Milnacipran for Depression
 A 2009 Cochrane review (16 trials, N = 2277 participants) compared milnacipran with other antidepressants for depression. Milnacipran was associated with fewer withdrawals because of adverse events (a measure of tolerability) compared with the TCAs (odds ratio [OR] 0.55), and weak evidence suggested fewer adverse events of sleepiness or drowsiness, dry mouth, or constipation versus these agents.
A 2009 Cochrane review (16 trials, N = 2277 participants) compared milnacipran with other antidepressants for depression. Milnacipran was associated with fewer withdrawals because of adverse events (a measure of tolerability) compared with the TCAs (odds ratio [OR] 0.55), and weak evidence suggested fewer adverse events of sleepiness or drowsiness, dry mouth, or constipation versus these agents.Noradrenergic and Specific Serotonergic Antidepressants (NaSSAs)
MOA (Mechanism of Action)
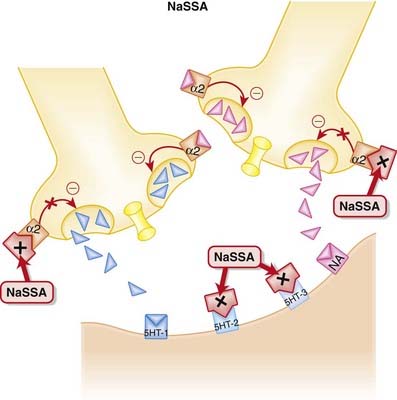
 NaSSAs have a dual mechanism of action:
NaSSAs have a dual mechanism of action: Inhibition of α2 autoreceptors and heteroreceptors
Inhibition of α2 autoreceptors and heteroreceptors• An autoreceptor is a receptor that when bound by ligand reduces release of that ligand into the synapse. The α2 receptor is a classic example of an autoreceptor, as when it is bound by noradrenaline (NA) it inhibits NA release.
• A heteroreceptor is like an autoreceptor, although when bound it can mediate the release of other neurotransmitters in addition to its own ligand.
• Thus inhibition of these autoreceptors and heteroreceptors prevents the negative feedback of NA on 5-HT and NA neurotransmission (Figure 23-4). Thus neurotransmission is sustained.
 Mirtazapine has a low affinity for muscarinic and dopaminergic receptors. However, it has high affinity for histamine (H1) receptors. These receptors all mediate many of the side effects of antidepressants, although dopamine receptors play a role in mood disorders.
Mirtazapine has a low affinity for muscarinic and dopaminergic receptors. However, it has high affinity for histamine (H1) receptors. These receptors all mediate many of the side effects of antidepressants, although dopamine receptors play a role in mood disorders.Side Effects
 Sedation occurs because of blockade of histamine receptors. It tends to predominate at lower doses, as increasing NA at higher doses counteracts this effect.
Sedation occurs because of blockade of histamine receptors. It tends to predominate at lower doses, as increasing NA at higher doses counteracts this effect. Increased appetite may be due to H1 antagonism, although the mechanism is unclear. Weight gain may be due to H1 antagonism, although the mechanism is unclear.
Increased appetite may be due to H1 antagonism, although the mechanism is unclear. Weight gain may be due to H1 antagonism, although the mechanism is unclear.Important Notes
 With its distinct mechanism, mirtazapine is seen as an alternative for patients intolerant to SSRIs. It has the advantage of lower incidence of sexual dysfunction, but it also has greater propensity for weight gain compared with SSRIs.
With its distinct mechanism, mirtazapine is seen as an alternative for patients intolerant to SSRIs. It has the advantage of lower incidence of sexual dysfunction, but it also has greater propensity for weight gain compared with SSRIs. Because of its sedative and appetite-stimulating effects, mirtazapine may theoretically be a more useful antidepressant in the elderly, as these patients often have depression accompanied by insomnia and weight loss.
Because of its sedative and appetite-stimulating effects, mirtazapine may theoretically be a more useful antidepressant in the elderly, as these patients often have depression accompanied by insomnia and weight loss. Although they are not thought of as being in the same class, the “atypical” antidepressants trazodone and nefazodone have some similarities to mirtazapine in their mechanisms of action. Both block 5-HT2 postsynaptic receptors and promote serotonin neurotransmission, although trazodone and nefazodone enhance serotonin by inhibiting its reuptake.
Although they are not thought of as being in the same class, the “atypical” antidepressants trazodone and nefazodone have some similarities to mirtazapine in their mechanisms of action. Both block 5-HT2 postsynaptic receptors and promote serotonin neurotransmission, although trazodone and nefazodone enhance serotonin by inhibiting its reuptake. Nefazodone is also believed to inhibit the reuptake of noradrenaline. Nefazodone was withdrawn from North American markets in the early 2000s for hepatotoxicity, and trazodone, once a very popular antidepressant, has been supplanted by newer agents such as the SSRIs.
Nefazodone is also believed to inhibit the reuptake of noradrenaline. Nefazodone was withdrawn from North American markets in the early 2000s for hepatotoxicity, and trazodone, once a very popular antidepressant, has been supplanted by newer agents such as the SSRIs. All antidepressants carry an FDA warning about increased suicidality, particularly in younger (<25 years of age) patients. The mechanism has not been established and there is not enough data to determine whether a lower risk exists for some antidepressants compared with others. These concerns must also be balanced against the potential for increased risk of completed suicides in untreated depression.
All antidepressants carry an FDA warning about increased suicidality, particularly in younger (<25 years of age) patients. The mechanism has not been established and there is not enough data to determine whether a lower risk exists for some antidepressants compared with others. These concerns must also be balanced against the potential for increased risk of completed suicides in untreated depression.Monoamine Oxidase Inhibitors (MAOIs)
Description
MAOIs are a heterogeneous group of agents that inhibit the monoamine oxidase (MAO) enzyme.