48 Prostate disease
Benign prostatic hyperplasia
Prostate cancer
Prostatitis
Benign prostatic hyperplasia
Epidemiology
BPH is seen in all races although the overall size of the prostate varies from race to race.
Pathophysiology
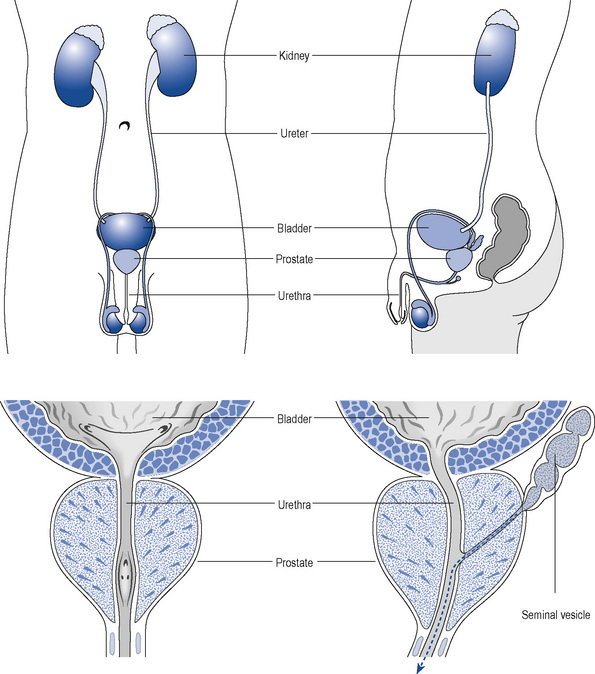
The prostate is a part glandular, part fibromuscular structure about the size of a walnut that surrounds the first part of the male urethra at the base of the bladder (Fig. 48.1). In simple terms, the prostate can be divided into a lobular inner zone encapsulated by an external layer. The inner zone is where benign hypertrophic changes are generally found, whereas most malignant changes originate in the peripheral zone.
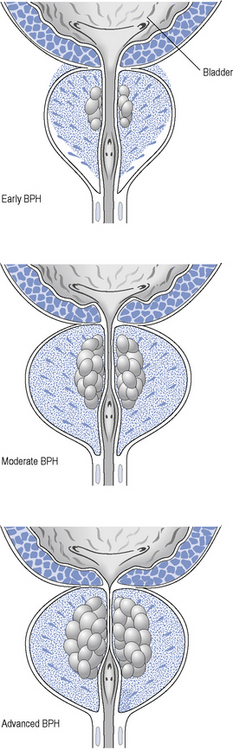
As the prostate enlarges, it can compress the urethra (Fig. 48.2) and this, together with increased adrenergic tone, can lead to bladder outflow obstruction (BOO) and lower urinary tract symptoms (LUTSs). Therefore, the term BPH includes benign prostatic enlargement (BPE), the clinical features associated with urinary obstruction and LUTSs.
Examination and investigations
Treatment
The British Association of Urological Surgeons has published guidelines for the management of BPH in primary care (Speakman et al., 2004), focussing on when urological referral is required and when non-invasive treatment can be initiated (Fig. 48.3).
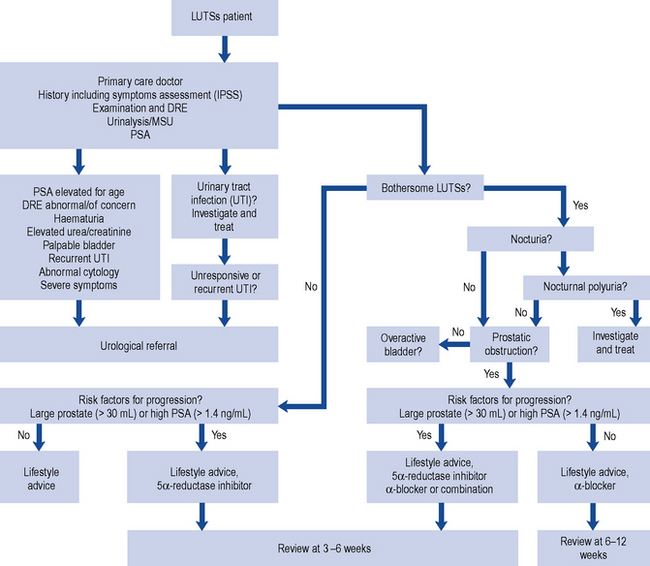
Fig. 48.3 Algorithm for the treatment of lower urinary tract symptoms (LUTSs) in males in primary care (from Speakman et al., 2004) (DRE, digital rectal examination; MSU, mid-stream urine sample; PSA, prostate-specific antigen).
Watchful waiting
Men with mild or moderate and not significantly bothersome LUTSs should be offered a trial of watchful waiting. This management strategy does not include any medical or surgical treatment but involves regular active monitoring. In some cases, symptoms remain unchanged for years and no further interventions are necessary since disease progression is minimal. Patients that adopt this modality should be offered education and lifestyle advice (Box 48.1) to manage their urological symptoms together with a review of their medication, particularly diuretics or other medicines known to affect the urinary system.
Therapeutic management
α-Adrenoceptor blocking drugs
Patients with BPH frequently experience erectile and ejaculatory dysfunction. The treatment of BPH should also aim to improve sexual function. However, the effect of α1-adrenoceptor antagonists on male sexual function is variable and influenced by the choice of agent and patient characteristics (Van Dijk et al., 2006).
Doxazosin
Doxazosin has a long half-life of about 22 h, which allows for once-daily dosing. When starting treatment, dose titration is recommended to limit postural hypotension. There would appear to be no significant difference in symptom score regardless of whether the standard or controlled-release preparation is used (Kirby et al., 2001).
Tamsulosin
Tamsulosin is a selective inhibitor of the α1A– and α1B-adrenoceptor. It has an elimination half-life of about 13 h and is available as a prolonged release formulation that allows once-daily dosing. There is no requirement to titrate the dose upward when initiating treatment. Although the side-effect profile of tamsulosin is similar to other α1-adrenoceptor antagonists, it is normally well tolerated (O’Leary, 2001). Intraoperative floppy iris syndrome (IFIS) has been reported during cataract surgery in men treated with tamsulosin, as it is highly selective to iris dilator muscle (Chaim et al., 2009). IFIS can lead to complications and poor outcomes during cataract surgery. As a result, it is essential that patients inform their cataract surgeon that they are taking tamsulosin during the pre-operative assessment. It has been recommended to avoid starting treatment and to discontinue treatment with tamsulosin 1–2 weeks before cataract surgery.
Alfuzosin
Alfuzosin displays a higher selectivity for the prostate compared with tamsulosin or doxazosin. It has a half-life of 5 h, but it is available as a once-daily formulation. It has a rapid onset of action and good tolerability (MacDonald and Wilt, 2005). It reduces the overall clinical progression of BPH and it appears to have a sustained beneficial effect on quality of life (Roehrborn, 2006). Alfuzosin has the least effect on ejaculatory function. Alfuzosin should not be co-administered with potent inhibitors of cytochrome P450 3A4 such as itraconazole, ketoconazole and ritonavir, since this can lead to a several fold increase to exposure in alfuzosin.
5α-Reductase inhibitors
The two agents currently available in this group are finasteride and dutasteride. Both have been shown to reduce prostate volume, to improve symptom scores and flow rates, and reduce the incidence of complications such as acute urinary retention (AUR) and the need for surgical intervention to treat BPH (Roehrborn et al., 2000, 2002). Improvements in LUTSs are normally seen after the first 6 months of treatment and are sustained during continuous treatment (Lam et al., 2003).
Combination therapy
The benefits of using a combination of doxazosin and finasteride compared to monotherapy have been demonstrated in over 3000 men (McConnell et al., 2003). Similarly, the combAT trial (Roehrborn et al., 2010) involved nearly 5000 men with moderate to severe symptoms of BPH and prostate enlargement treated with a combination of dutasteride and tamsulosin. This study demonstrated a significant improvement in BPH symptoms over a 4-year period when compared to either agent used alone. The combination was also found to reduce AUR and progression to BPH-related surgery and, although superior to tamsulosin with respect to these complications, combination therapy was not better than dutasteride. Overall, the adverse events associated with combination therapy were few and treatment was well tolerated.
Surgical treatments
Open prostatectomy
Open prostatectomy involves the surgical removal of an enlarged prostate. Typically, an incision is made through the lower abdomen although sometimes the incision is between the rectum and the base of the penis. This procedure is now performed infrequently and restricted to very enlarged prostate glands (larger than 100 mL) and those with large bladder stones or bladder diverticula (Stoevelaar and McDonnell, 2001). Open prostatectomy requires a longer hospital stay than transurethral resection and is associated with a higher incidence of bleeding and other complications.
Patient care
Patients generally seek medical help for BPH because of the impact of symptoms on their quality of life. Most men tolerate a high degree of symptoms and impact on daily activities before they seek help. Table 48.1 lists some common therapeutic problems in the management of BPH. Patients should receive information about the management options available, the investigations that they need to undergo and possible treatment outcomes and adverse effects. Patients receiving drug therapy should receive specific information about their treatment, including potential benefits, timeline of expected outcomes and possible side effects.
Table 48.1 Common therapeutic problems and proposed management strategies in benign prostatic hyperplasia
| Problem | Solution |
|---|---|
| Patient taking α-blocker still symptomatic after 2 weeks | Patients should be advised that it may take 2–6 weeks before symptomatic treatment relief is seen |
| Patient taking an α-adrenoceptor blocker complains of cardiovascular adverse effects such as dizziness, syncope, palpitations, tachycardia or angina | These side effects are more likely in elderly patients. They are most common after the first dose and reflect the hypotensive effects of the drugs. They can be reduced by titrating the dose or using more uroselective drugs such as tamsulosin |
| Sexual dysfunction | Decreased libido or impotence can occur in patients taking finasteride and dutasteride. Abnormal ejaculation can be caused by α-blockers. Tamsulosin in particular can cause a dry climax (retrograde ejaculation). Patients should be forewarned when discussing treatment options |
| Patient taking finasteride notices breast enlargement | Unilateral or bilateral gynaecomastia is a frequently reported side effect with finasteride and patients need to be counselled accordingly when discussing treatment options |
| Patient taking finasteride or dutasteride has a sexual partner who is pregnant | Exposure to semen should be avoided as both drugs can cause abnormalities to genitalia in a male fetus. The patient should be advised to use a condom |
There are two websites which produce particularly useful educational material on prostatic disease: the Men’s Health Forum (http://www.menshealthforum.org.uk/) and the Prostate Research Campaign (http://www.prostateuk.org/index.htm).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree