KEY CONCEPTS
![]() Prostate cancer is the most frequent cancer in men in the United States. African American ancestry, family history, and increased age are the primary risk factors for prostate cancer.
Prostate cancer is the most frequent cancer in men in the United States. African American ancestry, family history, and increased age are the primary risk factors for prostate cancer.
![]() Prostate-specific antigen is a useful marker to detect prostate cancer at early stages, predict outcome for localized disease, define disease-free status, and monitor response to androgen-deprivation therapy or chemotherapy for advanced-stage disease.
Prostate-specific antigen is a useful marker to detect prostate cancer at early stages, predict outcome for localized disease, define disease-free status, and monitor response to androgen-deprivation therapy or chemotherapy for advanced-stage disease.
![]() The prognosis for prostate cancer patients depends on the histologic grade, the tumor size, and the disease stage. More than 85% of patients with stage A1 disease but less than 1% of those with stage D2 can be cured.
The prognosis for prostate cancer patients depends on the histologic grade, the tumor size, and the disease stage. More than 85% of patients with stage A1 disease but less than 1% of those with stage D2 can be cured.
![]() Androgen deprivation therapy with a luteinizing hormone-releasing hormone (LHRH) agonist plus an antiandrogen should be used prior to radiation therapy for patients with locally advanced prostate cancer to improve outcomes over radiation therapy alone.
Androgen deprivation therapy with a luteinizing hormone-releasing hormone (LHRH) agonist plus an antiandrogen should be used prior to radiation therapy for patients with locally advanced prostate cancer to improve outcomes over radiation therapy alone.
![]() Androgen deprivation therapy, with either orchiectomy, an LHRH agonist alone or an LHRH agonist plus an antiandrogen (combined hormonal blockade), can be used to provide palliation for patients with advanced (stage D2) prostate cancer. The effects of androgen deprivation seem most pronounced in patients with minimal disease at diagnosis.
Androgen deprivation therapy, with either orchiectomy, an LHRH agonist alone or an LHRH agonist plus an antiandrogen (combined hormonal blockade), can be used to provide palliation for patients with advanced (stage D2) prostate cancer. The effects of androgen deprivation seem most pronounced in patients with minimal disease at diagnosis.
![]() Antiandrogen withdrawal, for patients having progressive disease while receiving combined hormonal blockade with an LHRH agonist plus an antiandrogen, can provide additional symptomatic relief. Mutations in the androgen receptor have been documented that cause antiandrogen compounds to act like receptor agonists.
Antiandrogen withdrawal, for patients having progressive disease while receiving combined hormonal blockade with an LHRH agonist plus an antiandrogen, can provide additional symptomatic relief. Mutations in the androgen receptor have been documented that cause antiandrogen compounds to act like receptor agonists.
![]() Chemotherapy, with docetaxel and prednisone improves survival in patients with castrate-refractory prostate cancer and is considered first-line therapy for these patients. Additional effective agents include cabazitaxel, enzalutamide, and abiraterone.
Chemotherapy, with docetaxel and prednisone improves survival in patients with castrate-refractory prostate cancer and is considered first-line therapy for these patients. Additional effective agents include cabazitaxel, enzalutamide, and abiraterone.
Prostate cancer is the most commonly diagnosed cancer in American men.1 For most men, prostate cancer has an indolent course, and treatment options for early disease include expectant management, surgery, or radiation. With expectant management, patients are monitored for disease progression or development of symptoms. Localized prostate cancer can be cured by surgery or radiation therapy, advanced prostate cancer is not yet curable. Treatment for advanced prostate cancer can provide significant disease palliation for many patients for several years after diagnosis. The endocrine dependence of this tumor is well documented, and hormonal manipulation to decrease circulating androgens remains the basis for the treatment of advanced disease.
EPIDEMIOLOGY
![]() Prostate cancer is the most frequent cancer among American men and represents the second leading cause of cancer-related deaths in all males.1 In the United States alone, it is estimated that 238,590 new cases of prostatic carcinoma were diagnosed and more than 29,720 men died from this disease in 2013.1 Although prostate cancer incidence increased during the late 1980s and early 1990s related to widespread prostate-specific antigen (PSA) screening, deaths from prostate cancer have been declining since 1995.1
Prostate cancer is the most frequent cancer among American men and represents the second leading cause of cancer-related deaths in all males.1 In the United States alone, it is estimated that 238,590 new cases of prostatic carcinoma were diagnosed and more than 29,720 men died from this disease in 2013.1 Although prostate cancer incidence increased during the late 1980s and early 1990s related to widespread prostate-specific antigen (PSA) screening, deaths from prostate cancer have been declining since 1995.1
ETIOLOGY
Table 108-1 summarizes the possible factors associated with prostate cancer.2,3 The widely accepted risk factors for prostate cancer are age, race-ethnicity, and family history of prostate cancer.2,3 The disease is rare in those younger than 40 years of age, but the incidence sharply increases with each subsequent decade, most likely because the individual has had a lifetime exposure to testosterone, a known growth signal for the prostate.3
TABLE 108-1 Risk Factors Associated with Prostate Cancer
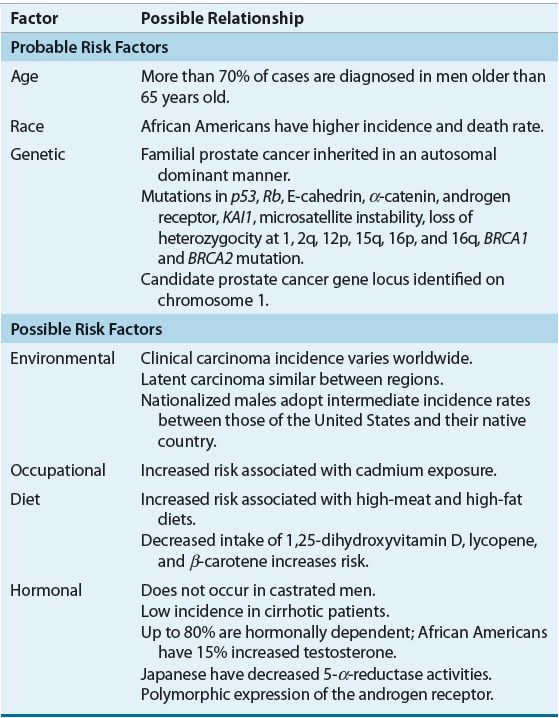
Race and Ethnicity
The incidence of clinical prostate cancer varies across geographic regions. Scandinavian countries and the United States report the highest incidence of prostate cancer, whereas the disease is relatively rare in Japan and other Asian countries.4 African American men have the highest rate of prostate cancer in the world, and in the United States, prostate cancer mortality in African Americans is more than twice that seen in white populations.1 Hormonal, dietary, and genetic differences, and differences in access to healthcare may contribute to the altered susceptibility to prostate cancer in these populations.2,3 Testosterone, commonly implicated in the pathogenesis of prostate cancer, is approximately 15% higher in African American men compared with white males. Activity of 5-α-reductase, the enzyme that converts testosterone to its more active form, dihydrotestosterone (DHT), in the prostate, is decreased in Japanese men compared with African Americans and whites.2,3 In addition, genetic variations in the androgen receptor exist. Activation of the androgen receptor is inversely correlated with CAG repeat length. Shorter CAG repeat sequences have been found in African Americans. Therefore the combination of increased testosterone and increased androgen receptor activation may account for the increased risk of prostate cancer for African American men.2,3 The Asian diet is generally considered to be low in fat and high in fiber with a high concentration of phytoestrogens, potentially explaining their decreased risk.4,5
Family History
Men with a brother or father with prostate cancer have twice the risk for prostate cancer as compared with the rest of the population.5 Familial clustering of a prostate cancer syndrome has been reported, and genome-wide scans have identified potential prostate cancer susceptibility candidate genes. Male carriers of germline mutations of BRCA1 and BRCA2 are known to have an increased risk for developing prostate cancer.6 Common exposure to environmental and other risk factors may also contribute to increased risk among patients with first-degree relatives with prostate cancer.5,7
Diet
Several epidemiologic studies support an association between high fat intake and risk of prostate cancer. A strong correlation between national per capita fat consumption and national prostate cancer mortality has been reported, and prospective case-control studies suggest that a high-fat diet doubles the risk of prostate cancer.5 This relationship between high fat intake and prostate cancer may explain differences in insulin-like growth factor-1 (IGF-1). High-calorie and high-fat diets stimulate hepatic production of IGF-1, which is involved in the regulation of proliferation and apoptosis of cancer cells.5 High levels of IGF-1 are associated with an increased risk for prostate cancer.5
Other dietary factors implicated in the development or prevention of prostate cancer include retinol, carotenoids, lycopene, and vitamin D consumption.5,7 Retinol, or vitamin A, intake, especially in men older than 70 years, is correlated with an increased risk of prostate cancer, whereas intake of its precursor, β-carotene, has a protective or neutral effect. Lycopene, obtained primarily from tomatoes, decreases the risk of prostate cancer in small cohort studies. Men who developed prostate cancer in one cohort study had lower levels of 1,25(OH)2-vitamin D than matched controls, although a prospective study did not support this. Clearly, dietary risk factors require further evaluation, and since fat and vitamins are modifiable risk factors, dietary intervention may be promising in prostate cancer prevention. Investigations of selenium and vitamin E supplementation are discussed further in the section titled chemoprevention.
Other Factors
Benign prostatic hyperplasia (BPH) is a common problem among elderly men, affecting more than 40% of men older than 70 years of age (see Chap. 67). BPH results in the urinary symptoms of hesitancy and frequency. Because prostate cancer affects a similar age group and often has similar presenting symptoms, the presence of BPH often complicates the diagnosis of prostate cancer, although it does not appear to increase the risk of developing prostate cancer.2,7
Smoking has not been associated with an increased risk of prostate cancer, but smokers with prostate cancer have an increased mortality resulting from the disease when compared with nonsmokers with prostate cancer (relative risk 1.5 to 2).2,7 In addition, in a prospective cohort analysis, alcohol consumption was not associated with the development of prostate cancer.
CHEMOPREVENTION
Currently, the most promising agents for the prevention of prostate cancer are the 5-α-reductase inhibitors, finasteride, and dutasteride.8–11 These drugs inhibit 5-α-reductase, an enzyme that converts testosterone to its more active form, DHT, which is involved in prostate epithelial proliferation. 5-α-reductase exists as two types, type I and type II, and both are implicated in the development of prostate cancer. Finasteride selectively inhibits the 5-α-reductase type II isoenzyme, whereas dutasteride inhibits both isoenzymes.9 Both finasteride and dutasteride falsely lower the PSA by approximately 50% in patients, and this must be considered when one interprets PSA in patients on these medications.12
The efficacy of 5-α-reductase inhibitors in reducing the risk of prostate cancer was recently evaluated in a Cochrane review.8 Eight randomized studies involving 41,638 men were included. Both the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) study, which compared dutasteride to placebo and included more than 8000 subjects and the Prostate Cancer Prevention Trial (PCPT), which compared finasteride to placebo and enrolled more than 18,000 subjects in the analysis. The mean subject age in the analysis was 64 years; 92% of subjects were white and 15% had a family history of prostate cancer. The mean (range) baseline PSA level was 3.1 (1.2 to 9.8 ng/mL [1.2 to 9.8 mcg/L]). Compared with placebo, 5-α-reductase inhibitors reduced the risk of prostate cancers detected by 25% (relative risk 0.75, 95% confidence interval [CI] 0.67–0.83; 1.4% absolute risk reduction [3.5% vs. 4.9%]). Studies were not designed to evaluate prostate cancer mortality, and in the combined analysis, 5-α-reductase inhibitors did not improve mortality.
Subjects who discontinued therapy or were lost to follow-up were not different between the placebo and treatment arms, but adverse effects, including gynecomastia, decreased libido, and erectile dysfunction, were more common in patients treated with 5-α-reductase inhibitors than in placebo. In the REDUCE trial, the incidence of “cardiac failure,” defined as congestive heart failure, cardiac failure, acute cardiac failure, ventricular failure, cardiopulmonary failure, or congestive cardiomyopathy, was greater in the dutasteride group (0.7%, n = 30) compared with the placebo group (0.4%, n = 16; P = 0.03), but deaths from cardiovascular events were not significantly different between groups.
The American Society of Clinical Oncology and the American Urological Association published a joint practice guideline for prostate cancer chemoprevention.13 The guideline recommends that asymptomatic men with a PSA ≤3.0 ng/mL (3.0 mcg/L) who are regularly screened with PSA for early detection of prostate cancer may benefit from a discussion of both the benefits of dutasteride or finasteride for 7 years for the prevention of prostate cancer and the potential risks.13
The guideline does not recommend the use of finasteride or dutasteride for prostate cancer chemoprevention and noted that, while most panel members believed the higher risk of high-grade cancer in the finasteride group observed in the PCPT is most likely related to biases, cancer induction or promotion by finasteride cannot be excluded with certainty. In addition, while finasteride and dutasteride reduce the prevalence of prostate cancer, the impact of 5-α-reductase inhibitors on prostate cancer morbidity and mortality has not been demonstrated. Patients considering finasteride or dutasteride for prostate cancer chemoprevention or taking it for benign conditions such as BPH must weigh the risks and benefits of treatment. The primary benefit is that these agents reduce the incidence of prostate cancer by about 25%, and improve lower urinary tract symptoms of BPH, but the risks include the potential for more high-grade prostate cancers; the long-term benefit of these agents is not known; and reversible sexual adverse effects can occur.13
Selenium and vitamin E alone or in combination were evaluated in the Selenium and Vitamin E Cancer Prevention Trial (SELECT), a clinical trial investigating their effects on the incidence of prostate cancer in healthy men. The data and safety monitoring committee found that after 5 years selenium or vitamin E taken alone or together did not prevent prostate cancer. Based on these data and safety concerns, the trial was halted. With longer follow-up of that trial, dietary supplementation with vitamin E significantly increased the risk of prostate cancer by 17% (P = 0.008).14 Other agents, including vitamin D, lycopene, green tea, nonsteroidal antiinflammatory agents, isoflavones, and statins, are under investigation for prostate cancer and show promise; however, none are currently recommended for routine use outside of a clinical trial.15
SCREENING
Digital rectal examination (DRE) has been recommended since the early 1900s for the detection of prostate cancer. The primary advantage of DRE is its specificity, reported at greater than 85%, for prostate cancer. Other advantages of DRE include low cost, safety, and ease of performance. However, DRE is relatively insensitive and is subject to interobserver variability. DRE as a single screening method has poor compliance and showed little effect in preventing metastatic prostate cancer in one large observational study.16
![]() PSA is a useful marker for detecting prostate cancer at early stages, predicting outcome for localized disease, defining disease-free status, and monitoring response to androgen-deprivation therapy or chemotherapy for advanced-stage disease. PSA is used widely for prostate cancer screening in the United States, with simplicity its major advantage and low specificity its primary limitation.17 PSA may be elevated in men with acute urinary retention, acute prostatitis, and prostatic ischemia or infarction, as well as BPH, a nearly universal condition in men at risk for prostate cancer. PSA elevations between 4.1 and 10 ng/mL (4.1 and 10 mcg/L) cannot distinguish between BPH and prostate cancer, limiting the utility of PSA alone for the early detection of prostate cancer. Additionally, many men with clinically significant prostate cancer do not have a serum PSA outside the reference range.18
PSA is a useful marker for detecting prostate cancer at early stages, predicting outcome for localized disease, defining disease-free status, and monitoring response to androgen-deprivation therapy or chemotherapy for advanced-stage disease. PSA is used widely for prostate cancer screening in the United States, with simplicity its major advantage and low specificity its primary limitation.17 PSA may be elevated in men with acute urinary retention, acute prostatitis, and prostatic ischemia or infarction, as well as BPH, a nearly universal condition in men at risk for prostate cancer. PSA elevations between 4.1 and 10 ng/mL (4.1 and 10 mcg/L) cannot distinguish between BPH and prostate cancer, limiting the utility of PSA alone for the early detection of prostate cancer. Additionally, many men with clinically significant prostate cancer do not have a serum PSA outside the reference range.18
Early detection of potentially curable prostate cancers is the goal of prostate cancer screening. For cancer screening to be beneficial, it must reliably detect cancer at an early stage, when intervention would decrease mortality. Whether prostate cancer screening, with PSA, DRE or a combination fits these criteria has generated considerable controversy, and two recent studies have done little to resolve the controversy.19–22 The European Randomized Study of Screening for Prostate Cancer (ERSPC) evaluated the effect of PSA screening on prostate cancer mortality. More than 182,000 men from seven different European countries were randomized between being offered screening with PSA to no screening. The frequency of screening and PSA threshold for a biopsy varied by country. Most centers used a PSA cutoff of 3 ng/mL (3 mcg/L), but Belgium allowed up to 10 ng/mL (10 mcg/L). Most centers screened every 4 years, although Sweden screened every 2 years. Eighty-two percent of men in the screening group had at least one PSA performed. With a median follow-up of 11 years, the cumulative incidence of prostate cancer was 9.6% in the screening group and 6.0% in the control group.23 The rate ratio for death from prostate cancer in the screening group, compared with the control group, was 0.79 (95% CI, 0.68 to 0.91; adjusted P = 0.001), which corresponds to about one less death from prostate cancer per 1000 men (at a median follow-up of 11 years) in the screened group compared with the unscreened group. Of the 136,689 PSA tests performed, 16.6% of the tests were positive; biopsies were performed for 86% of men with elevated PSAs. Overall mortality was similar in the two study groups (rate ratio 0.99, 95% CI, 0.97 to 1.01).23
In the United States, the Prostate, Lung, Colon and Ovarian Screening (PLCO) study randomized 76,693 men to receive either annual screening (38,343 subjects) or usual care as the control (38,350 subjects). In the screening group, men were offered annual PSA testing for 6 years and DRE for 4 years. Compliance with screening was 85%. Men in the usual care group were able to receive screening, with the rate of PSA testing ranging from 40% to 52% and DRE from 41% to 46%. After 13 years of follow-up, the incidence of death per 10,000 person-years was not significantly different between the two groups with 3.7 (158 deaths total) in the screening group and 3.4 (145 deaths total) in the control group (relative risk, 1.09; 95% CI, 0.87 to 1.36).24
In the United States, clinicians believe that neither DRE nor PSA is sensitive or specific enough to be used alone as a screening test. Although the relative predictability of DRE and PSA is similar, the tumors identified by each method are different. The common approach to prostate cancer screening today involves offering a baseline PSA and DRE at age 40 years with annual evaluations beginning at age 50 to all men of normal risk with a 10-year or greater life expectancy. Men with an increased risk of prostate cancer, including men of African American ancestry and men with a family history of prostate cancer, may begin screening earlier, at age 40 to 45 years.
Despite this common practice, the benefits of prostate cancer screening remain controversial.19,20,22 The ERSPC demonstrated that PSA testing every 4 years was better than no PSA testing, decreasing prostate cancer deaths in the screened group by about 1 per 1,000 men screened compared with the unscreened group, but the false-positive rate was 76%, resulting in more than 13,000 unnecessary biopsies. The PLCO screening study showed no reduction in prostate cancer death between the annual (PSA and DRE) screening group and the usual care group, which is not surprising given the small reduction in death expected and that about one-half of the patients in the usual screening groups had PSA and DRE screening performed. Both studies demonstrated that screening identifies more prostate cancers than not screening.21,23,24 PSA measurements can identify small, subclinical prostate cancers, where no intervention may be required. Detecting prostate cancer in those not needing therapy not only increases the cost of care through unnecessary screening and workups, but also increases harm by subjecting some patients to unnecessary therapy. Based on this evidence, the United States Preventative Services Task Force (USPSTF) recommends against screening for prostate cancer (grade D recommendation), based on moderate or high certainty that screening has no net benefit or that the harms outweigh the benefits.21,22 The American Cancer Society recommends that asymptomatic men who have at least a 10-year life expectancy have an opportunity to make to make an informed decision about prostate cancer screening, including discussion of the uncertainties, risks, and potential benefits associated with screening.20
Clinical Controversy…
Based on the available evidence, Gulati et al. recently evaluated the comparative effectiveness of alternative PSA screening strategies.25 Examples of alternative screening strategies include the use of higher PSA thresholds for biopsy referral or longer screening intervals. Several of the screening scenarios were predicted to produce similar reductions in prostate cancer mortality and reduce harms.
PATHOPHYSIOLOGY
The prostate gland is a solid, rounded, heart-shaped organ positioned between the neck of the bladder and the urogenital diaphragm (Fig. 108-1). The normal prostate is composed of acinar secretory cells arranged in a radial shape and surrounded by a foundation of supporting tissue. The size, shape, or presence of acini is almost always altered in the gland that has been invaded by prostatic carcinoma. Adenocarcinoma, the major pathologic cell type, accounts for more than 95% of prostate cancer cases.26,27 Much rarer tumor types include small cell neuroendocrine cancers, sarcomas, and transitional cell carcinomas.
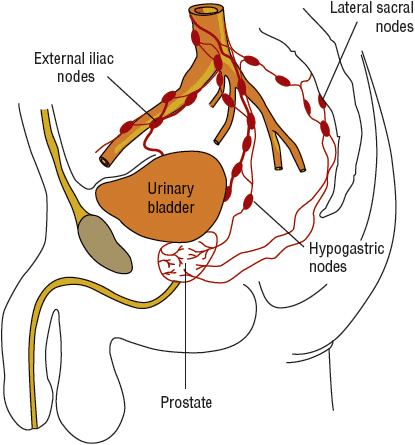
FIGURE 108-1 The prostate gland.
Prostate cancer can be graded systematically according to the histologic appearance of the malignant cell and then grouped into well, moderately, or poorly differentiated grades.27,28 Gland architecture is examined and then rated on a scale of 1 (well differentiated) to 5 (poorly differentiated). Two different specimens are examined, and the score for each specimen is added. Groupings for total Gleason score are 2 to 4 for well differentiated, 5 or 6 for moderately differentiated, and 7 to 10 for poorly differentiated tumors. Poorly differentiated tumors grow rapidly (poor prognosis), while well-differentiated tumors grow slowly (better prognosis).
Metastatic spread can occur by local extension, lymphatic drainage, or hematogenous dissemination.28,29 Lymph node metastases are more common in patients with large, undifferentiated tumors that invade the seminal vesicles. The pelvic and abdominal lymph node groups are the most common sites of lymph node involvement (see Fig. 108-1). Skeletal metastases from hematogenous spread are the most common sites of distant spread. Typically, the bone lesions are osteoblastic or a combination of osteoblastic and osteolytic. The most common site of bone involvement is the lumbar spine. Other sites of bone involvement include the proximal femurs, pelvis, thoracic spine, ribs, sternum, skull, and humerus. The lung, liver, brain, and adrenal glands are the most common sites of visceral involvement, although these organs are not usually initially involved. About 25% to 35% of patients will have evidence of lymphangitic or nodular pulmonary infiltrates at autopsy. The prostate is rarely a site for metastatic involvement from other solid tumors.
Normal growth and differentiation of the prostate depend on the presence of androgens, specifically DHT.29,30 The testes and the adrenal glands are the major sources of circulating androgens. Hormonal regulation of androgen synthesis is mediated through a series of biochemical interactions between the hypothalamus, pituitary, adrenal glands, and testes (Fig. 108-2). Luteinizing hormone-releasing hormone (LHRH) released from the hypothalamus stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary gland. LH complexes with receptors on the Leydig cell testicular membrane and stimulates the production of testosterone and small amounts of estrogen. FSH acts on the Sertoli cells within the testes to promote the maturation of LH receptors and to produce an androgen-binding protein. Circulating testosterone and estradiol influence the synthesis of LHRH, LH, and FSH by a negative feedback loop operating at the hypothalamic and pituitary level.31 Prolactin, growth hormone, and estradiol appear to be important accessory regulators for prostatic tissue permeability, receptor binding, and testosterone synthesis.
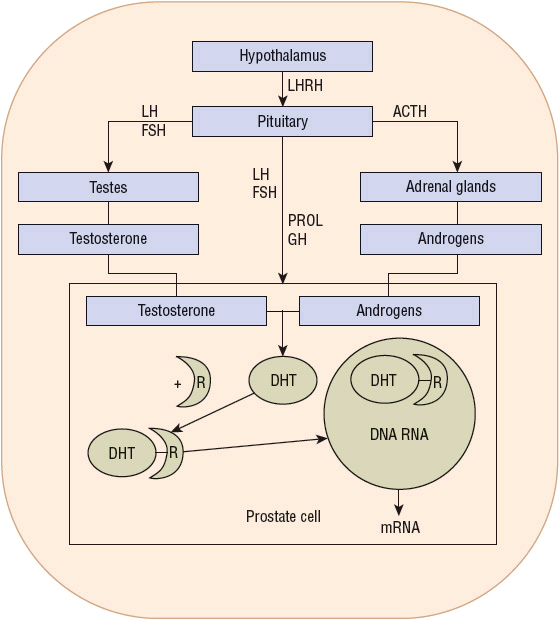
FIGURE 108-2 Hormonal regulation of the prostate gland. (ACTH, adrenocorticotropic hormone; DHT, dihydrotestosterone; FSH, follicle-stimulating hormone; GH, growth hormone; LH, luteinizing hormone; LHRH, luteinizing hormone-releasing hormone; PROL, prolactin; R, receptor).
Testosterone, the major androgenic hormone, accounts for 95% of the androgen concentration. The primary source of testosterone is the testes, but 3% to 5% of the testosterone concentration is derived from direct adrenal cortical secretion of testosterone or C19 steroids such as androstenedione.28–30
In early-stage prostate cancers, aberrant tumor cell proliferation is promoted by the presence of androgens. For these tumors, blockade of androgens induces tumor regression in most patients. Hormonal manipulations to ablate or reduce circulating androgens can occur through several mechanisms29,30 (Table 108-2). The organs responsible for androgen production can be removed surgically (orchiectomy, hypophysectomy, or adrenalectomy). Hormonal pathways that modulate prostatic growth can be interrupted at several steps (see Fig. 108-2). Interference with LHRH or LH can reduce testosterone secretion by the testes (estrogens, LHRH agonists, progestogens, and cyproterone acetate). Estrogen administration reduces androgens by directly inhibiting LH release, by acting directly on the prostate cell, or by decreasing free androgens by increasing steroid-binding globulin levels.28–30
TABLE 108-2 Hormonal Manipulations in Prostate Cancer
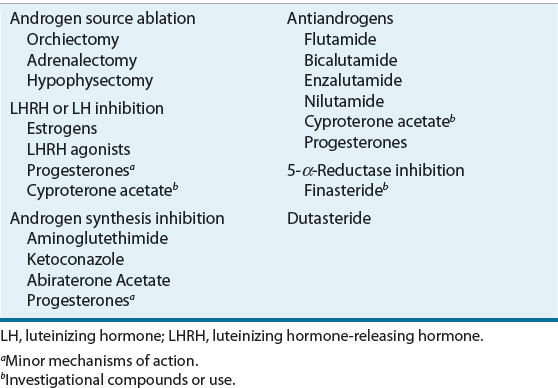
Isolation of the naturally occurring hypothalamic decapeptide hormone luteinizing hormone-releasing hormone or LHRH has provided another group of effective agents for advanced prostate cancer treatment. The physiologic response to LHRH depends on both the dose and the mode of administration. Intermittent pulsed LHRH administration, which mimics the endogenous release pattern, causes sustained release of both LH and FSH, whereas high-dose or continuous IV administration of LHRH inhibits gonadotropin release due to receptor downregulation.23 Structural modification of the naturally occurring LHRH and innovative delivery have produced a series of LHRH agonists that cause a similar downregulation of pituitary receptors and a decrease in testosterone production.31
Androgen synthesis can also be inhibited in the testes or in the adrenal gland. Aminoglutethimide inhibits the desmolase-enzyme complex in the adrenal gland, thereby preventing the conversion of cholesterol to pregnenolone. Pregnenolone is the precursor substrate for all adrenal-derived steroids, including androgens, glucocorticoids, and mineralocorticoids. Ketoconazole, an imidazole antifungal agent, causes a dose-related reversible reduction in serum cortisol and testosterone concentration by inhibiting both adrenal and testicular steroidogenesis.31 Megestrol is a synthetic derivative of progesterone that exhibits a secondary mechanism of action by inhibiting androgen synthesis. This inhibition appears to occur at the adrenal level, but circulating levels of testosterone are also reduced, suggesting that inhibition at the testicular level may also occur.31
Antiandrogens inhibit the formation of the DHT-receptor complex and therefore interfere with androgen activity at the cellular level.31 The conversion of testosterone to DHT may be inhibited by 5-α-reductase inhibitors.7
In advanced stages of disease, prostate cancer cells may be able to survive and proliferate without the signals normally provided by circulating androgens.31 When this occurs, the tumor is no longer sensitive to therapies that depend on androgen blockade. These tumors are often referred to as hormone refractory or androgen independent.
CLINICAL PRESENTATION
Prior to the implementation of routine screening, prostate cancers were frequently identified on the investigation of symptoms, including urinary hesitancy, retention, painful urination, hematuria, and erectile dysfunction. With the introduction of screening techniques, most prostate cancers are now identified prior to the development of symptoms.



