Chapter 4 Processing, extraction and purity
EXTRACTION FROM NATURAL PRODUCTS
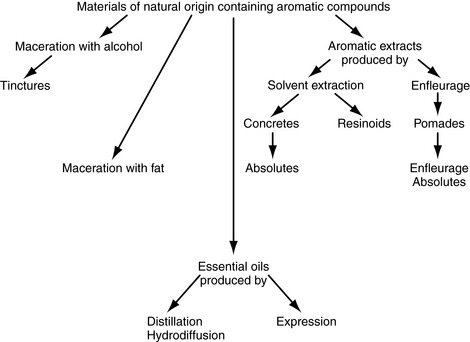
When considering quality and composition of an essential oil the method of extraction plays a crucial role. An essential oil is that oil extracted from plant material that is volatile at room temperature, but there are a number of other products whose nature and means of production need to be clarified – absolutes, resinoids, tinctures, floral waters, etc. (see Fig. 4.1).
The efficiency and yield of oil will depend on the method used and is usually reflected in price.
Distillation
Water distillation
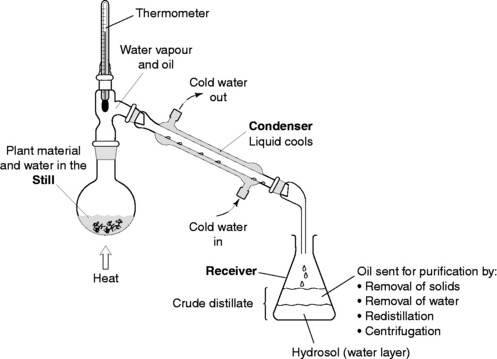
The distillation apparatus, commonly called a ‘still’, consists of a vessel for plant material and water, a condenser to cool and condense the vapour produced and a method of collection, or ‘receiver’. Material from the appropriate part of the plant for extraction is immersed in water in the distillation vessel. This is then heated to boiling point and the steam (water vapour) carries out the volatile oils. The water safeguards some components by preventing overheating as the temperature will not exceed 100 °C (the boiling point of water at normal pressure). However, the distillation can be a long process and the water may damage some other compounds.
AROMAFACT
Essential oils with a high percentage of esters can become hydrolyzed (hydrolysis is a chemical reaction of a substance with water) by contact with the hot water, which breaks them down to their constituent alcohols and carboxylic acids (see Ch. 3, esters). In lavender, linalyl acetate can break down into linalool and acetic acid, so a short distillation time is favourable.
The extraction of essential oils from plant material can be easily carried out in the chemistry laboratory using the apparatus shown in Figure 4.2.
Steam distillation
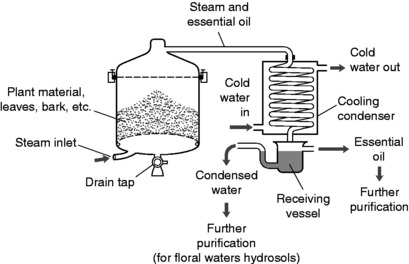
In steam distillation, steam – which is water vapour – is passed through the plant material at high pressure. Constituents that are insoluble in the water but volatile enough to be driven off by the steam come over and are cooled, condensed and collected in the receiving vessel.
The resultant liquid is a mixture of immiscible oil and water, which separate out. Steam distillation is economical in processing large amounts of material, requiring little labour or complex extraction apparatus. A simple industrial steam distillation setup is shown in Figure 4.3.
Steam distillation is quick, which minimizes damage to the compounds in the essential oil. The technique is good for extraction of volatile compounds from the monoterpenes (10 carbon atoms) to the diterpenes (20 carbon atoms).
Solvent extraction
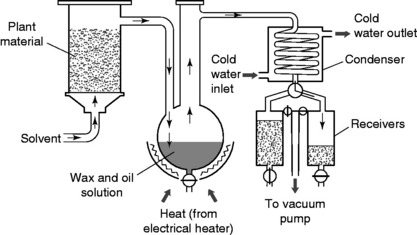
Aromatic plant material is placed into organic solvents such as acetone (propanone) or hexane, which dissolve out the oils. Other solvents used are methanol, ethanol, toluene and petroleum ether. In some processes the plant material is broken up, to aid penetration of solvent into the tissues, by placing it in a rotating drum with internal blades to ensure thorough mixing. The materials that become dissolved include not only the essential oil but also natural waxes, resinous materials, chlorophyll and other pigments.
The residue obtained is repeatedly washed with fresh amounts of the same solvent to maximize yield. Solvent is then recovered in a still at reduced pressure, which lowers the solvent’s boiling point and permits the use of gentle heat. The concentrated extract is not distilled but is retained in the vessel in a liquid state. When it is removed and cooled, the concentrated extract solidifies to a waxy consistency called a concrete, which is made up of approximately 50% odourless wax. The unwanted wax is removed by washing with alcohol, which extracts the essential oil. The alcohol mixture is then filtered and alcohol is removed by vacuum distillation. The final residue is called the absolute. A typical solvent extraction plant is shown in Fig. 4.4; in this system the solvent is pumped through a bed of the plant material.
If the residue from the initial extraction is of a resinous nature, it is called a resinoid, e.g. benzoin, myrrh, frankincense. Many resinoids will yield essential oils when distilled if they contain sufficiently volatile aromatic constituents.