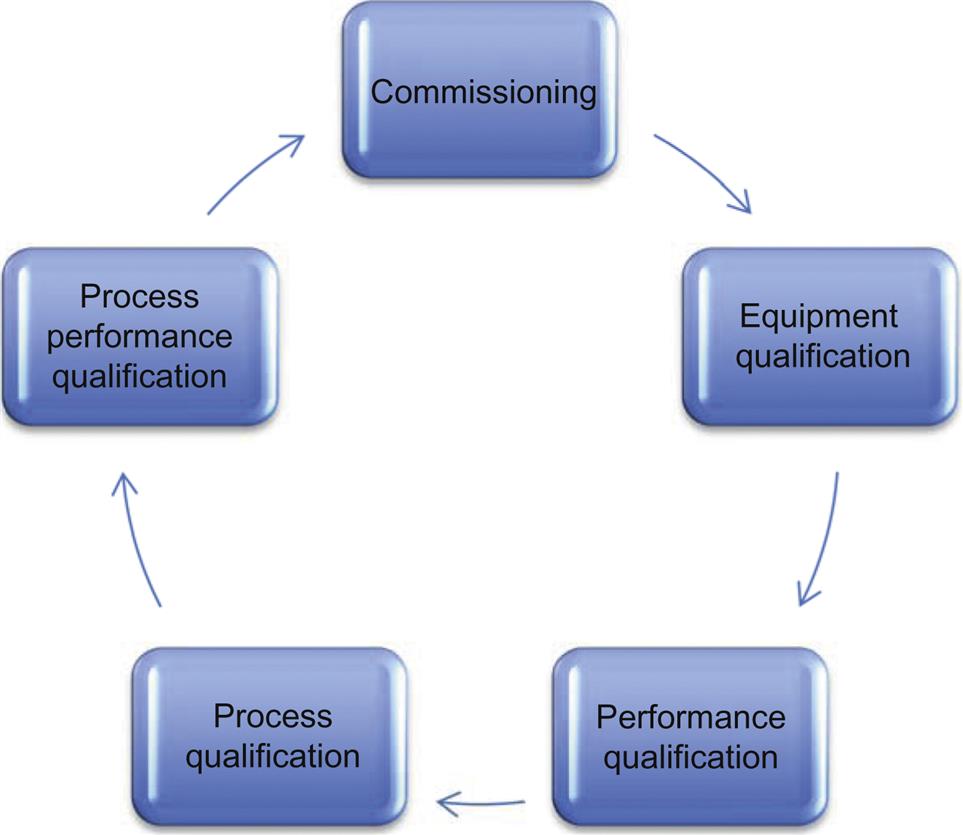
It must be understood that maintaining compliance is a never-ending process. PV is not one step, thus it really isn’t the “last step” in the compliance program (refer to Fig. 1.2) meeting the compliant state is a circle (thus a life cycle approach is needed). There are many pieces that need to come together before it can be considered complete. While this book is about developing a successful PV, other areas that support or otherwise contribute to the PV need to be considered. For example, cleaning validation or the qualification of the facility are critical components of the PV. A manufacturer cannot just run their process without first assuring that all of the equipment, utilities, facility, and supporting processes are fully qualified or validated.11 These are discussed in the later chapters.
The facility must be capable of producing the product (ie, its location, size, design). The equipment and utilities supporting the process need to be qualified and shown to meet the criteria needed to consistently produce the product (system suitability) and the operators need training on the process and use of the equipment. And lastly, but certainly not least, all aspects of 21 CFR 211 (GMPs for Finished Pharmaceuticals) must be met.
The CGMPs are written so as to allow the manufacturer the ability to control their own process. As you will find in Chapter 2, “A Brief Review of the Regulations,” the regulations in Title 21 PART 211 of the Code of Federal Regulations (CFR) or in the Food, Drug, and Cosmetic Act (FD&C)12 are not specific for PV. However, all of the rules apply even to non-pharmaceutical products (eg, biologics, devices, etc.). There are parts of the CFR that apply to other types of products yet Part 211 applies to all at least to some degree. Thus, if the manufacturing process is to produce a combination product Part 211 and the specific Part for the combination product (eg, a stent containing a drug would follow 21 CFR 211 and 820) will be applicable.
There are four types of validation that can be performed. These are:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree