FIGURE 28-1 Clotting cascade depicting site of action of factor Xa and thrombin inhibitors. (Used with permission of Erik Abel, PharmD, BCPS.)
TABLE 28-1 Pharmacokinetic Properties of Novel Anticoagulants
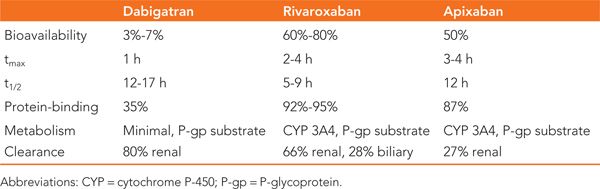
TABLE 28-2 Serious Drug Interactions of Novel Anticoagulants
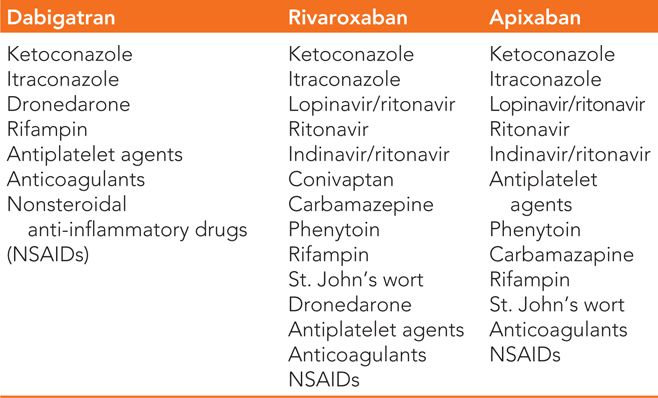
TABLE 28-3 Advantages and Disadvantages of Anticoagulants for Prevention of Thromboembolism in Patients with Atrial Fibrillation
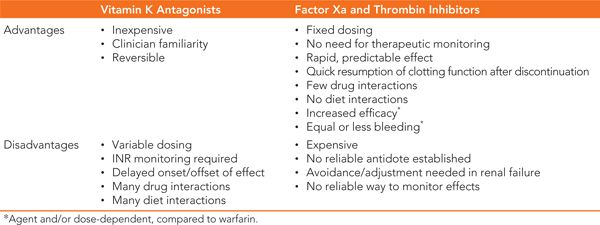
Dabigatran (Pradaxa) is the first direct thrombin inhibitor available for thromboembolic prevention in nonvalvular AF. Dabigatran directly blocks free and clot-bound thrombin (Factor II) in the final step of the clotting cascade (See Figure 28-1). Similar to factor Xa inhibitors, mechanistic and pharmacokinetic properties of dabigatran offer many advantages compared to warfarin (see Tables 28-1 to 28-3).
Similar to warfarin, the primary adverse effect of factor Xa and thrombin inhibitors is increased risk of bleeding. Though clinical trials suggest similar or lower rates of bleeding compared to warfarin, management of severe bleeding presents more challenges with the new agents. Poor sensitivity of clinically available laboratory tests (PT, INR, aPTT) and lack of a specific antidote for the new agents make assessment and reversal of the anticoagulant effect difficult in the setting of acute hemorrhage.
CLINICAL TRIALS
In patients with AF, each of the new anticoagulants has been compared to warfarin for stroke and systemic embolism prevention in large, randomized, noninferiority trials. Dabigatran was assessed by the RE-LY trial, rivaroxaban in the ROCKET-AF trial, and apixaban in the ARISTOTLE study.5–7 Notable similarities of these trials include:
• Large sample sizes
• Common end points—combination of stroke and systemic embolism (efficacy) and similarly defined major bleeding (safety)
• Exclusion of patients with severe renal insufficiency, significant valvular heart disease, or prosthetic heart valves
• Duration of follow-up of about 2 years
Significant differences in the design of these trials include:
• Patients randomized to warfarin were unblinded to treatment in RE-LY but were blinded in ROCKET-AF and ARISTOTLE.
• Adjustments for renal insufficiency were made in ROCKET-AF and ARISTOTLE, but not in RE-LY.
• Higher stroke-risk patients (CHADS2 ≥2) were enrolled in the ROCKET-AF trial.
In RE-LY, patients were randomized to one of 2 doses of dabigatran (110 mg or 150 mg twice daily) or open-label warfarin with an INR goal of 2 to 3. Time in therapeutic INR range for the warfarin group was roughly 65%. Approximately two-thirds of the patients were considered high risk for stroke (CHADS2 ≥2). For the prevention of stroke and systemic embolism, the results of RE-LY demonstrate noninferiority for both doses of dabigatran compared to warfarin as well as superiority for the 150 mg dose (Table 28-4). Compared with warfarin, major bleeding was reduced with the 110 mg dose and similar with the 150 mg dose (Table 28-5). Of note, both doses of dabigatran showed reduced rates of life-threatening bleeding and intracranial hemorrhage versus warfarin while the 150 mg dose was associated with an increased rate of gastrointestinal (GI) bleeding.
TABLE 28-4 Intention-to-Treat Efficacy Results of the Major Clinical Trials of New Oral Anticoagulants Compared to Warfarin for the Combined Primary Endpoint of Stroke or Systemic Embolism5,6,7
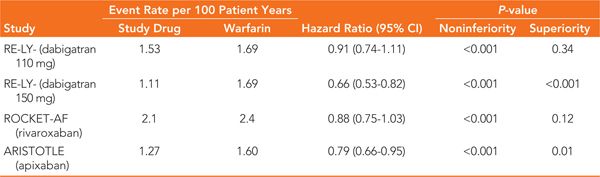
TABLE 28-5 Bleeding Endpoint Results of the Major Clinical Trials of New Oral Anticoagulants Compared to Warfarin5,6,7
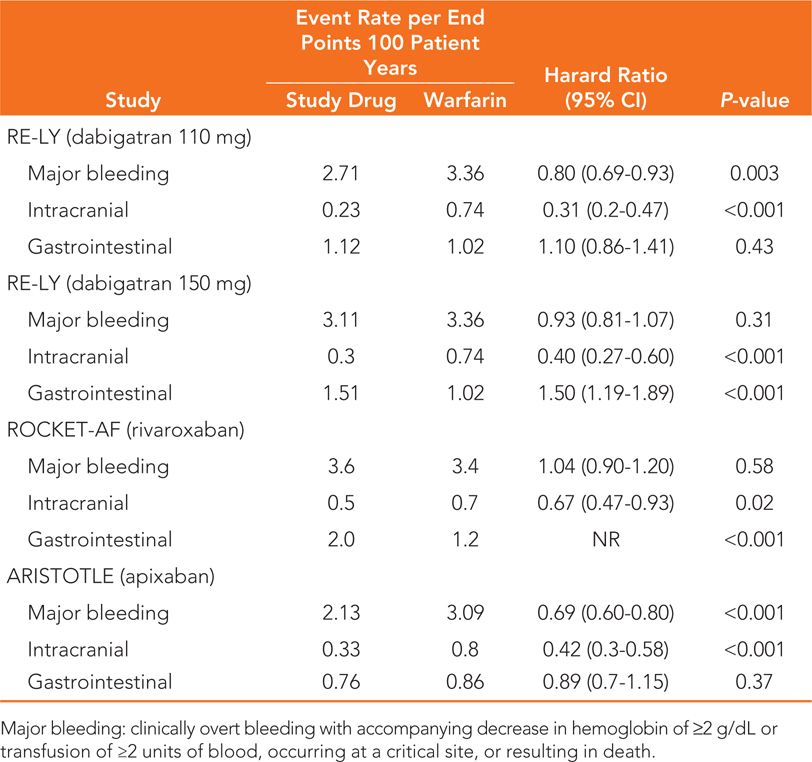
The ROCKET-AF study compared rivaroxaban 20 mg daily (15 mg daily for CrCl 30 to 49 mL/min) with blinded adjusted-dose warfarin (goal INR 2 to 3). Warfarin patients had INR values within the therapeutic range 55% of the time. Patients at low or intermediate risk of stroke (CHADS2 0 to 1) were excluded from ROCKET-AF. The primary outcome of this study by the intention-to-treat analysis revealed that rivaroxaban was noninferior to warfarin for the prevention of stroke and systemic embolism in this patient population (see Table 28-4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


