Preparedness for A Bioterrorist Attack With Smallpox
Whitni B. Davidson
Andrea M. McCollum
Inger K. Damon
Smallpox is an infectious disease caused by an orthopoxvirus, variola virus, and efforts of a worldwide program led to its eradication. Historical accounts have long placed smallpox as the cause of many epidemics and deaths. Smallpox has a high interhuman transmission rate, multiple transmission routes, and a high case fatality rate. Jenner’s inoculation with cowpox in 1798 demonstrated that protection against smallpox could be achieved using dermal infection with a related orthopoxvirus. Shortly thereafter, vaccination with live vaccinia virus was introduced as an individual and population-based method to prevent infection with smallpox. An extensive global campaign including vaccination, surveillance, containment, and infection control practices was led by the World Health Organization (WHO) beginning in 1967. The last case of “natural” smallpox was reported in a Somalian patient in 1977, and two persons were infected as a result of a laboratory exposure in 1978. Worldwide eradication was pronounced in 1980. Childhood vaccination programs ceased before, or shortly after, the declaration of disease eradication.
Smallpox, as known in the historical medical literature, did not have an animal reservoir; thus, there is no risk of “natural” human infections appearing again, and this, in part, contributed to the ability to eradicate the disease. However, the virus itself has not been eradicated. Declared stocks of the virus are securely maintained in two WHO reference laboratories, one at the U.S. Centers for Disease Control and Prevention (CDC) and the other at the State Research Center of Virology and Biotechnology (Vektor Institute) in Russia. There is some belief that undeclared stocks may also exist (1). Thus, the chance of an accidental or intentional release of smallpox is not zero. This, along with an increasing susceptible, unvaccinated, mobile population, causes great concern about the dangers posed by variola virus in the world today.
Large-scale public health efforts have resulted in the development of emergency plans, response guidelines, and acquisition of vaccine stocks. For example, the United States has enough smallpox vaccine in its stockpile to vaccinate each U.S. citizen in the event of a release of the virus. This chapter will review the biology and epidemiology of smallpox, vaccination information including adverse events, and hospital control and prevention of transmission of the disease.
VIROLOGY AND PATHOLOGY
Variola virus belongs to the Poxviridae family, subfamily Chordopoxvirinae, as a member species of the Orthopoxvirus genus. Chordopoxviruses infect a wide variety of animals including birds, rodents, ruminants, and humans, and these viruses can exhibit wide to narrow “host” species specificities and host ranges. The Orthopoxvirus genus includes four virus species known to infect humans, variola, monkeypox, vaccinia, cowpox, as well as others not currently known to naturally infect humans. Orthopoxviruses are closely related and immunologically cross-reactive. Edward Jenner demonstrated cross-protection against variola in humans first using cowpox in 1798 and then using vaccinia virus. Poxviruses have large virions, approximately 140 to 260 nm × 220 to 450 nm. Oval-or brick-shaped virions encapsulate linear, double-stranded DNA genomes of approximately 200 kb in length. There are two epidemiologically characterized variants of variola infection: “variola major” and “variola minor”; each differs in case fatality rates and some viruses associated with the less severe disease manifestations can be distinguished in the laboratory or by genetic markers (2).
Much of the information about smallpox pathogenesis has been gleaned from using animal models with a variety of orthopoxvirus challenges. In human smallpox disease, epidemiologic information indicated that the common route for infection was via the respiratory tract; transmission via the skin or congenitally occurred less frequently. The virus asymptomatically replicates in the endothelium and enters the reticuloendothelial system. Additional replication occurs in the lymph nodes. Macrophages migrate to infected lymph nodes early in infection, and the production of cytotoxic T cells and B cells limits the spread of infection. Neutralizing antibodies can be found during the first week of infection. Secondary viremia followed by initial onset of symptoms occurs on average 12 days after transmission. Hemagglutination inhibition and complement fixation antibodies are present approximately 16 and 18 days postinfection, respectively. These antibodies may dissipate after 1 year; however, neutralizing antibodies are present for many years postinfection (2, 3 and 4). Currently, humoral immune responses to orthopoxvirus infections are more commonly measured
via immunoglobulin M and/or G enzyme-linked immunosorbent assays and neutralizing responses (5).
via immunoglobulin M and/or G enzyme-linked immunosorbent assays and neutralizing responses (5).
A rash develops over the entire body and goes through several stages (described below) as macrophages migrate to the epidermis. Aside from oropharynx and skin lesions, virus can be found in lymph nodes, bone marrow, spleen, liver, kidney, urine, and conjunctival secretions (2,3). In fact, virtually all organs are affected. Endothelial cells lining the sinusoids of the liver swell and can become necrotic, and parenchymal cells swell. The spleen enlarges with increased lymph involvement. Hemorrhaging of renal, gastric, and pharyngeal membranes and endocardium and myocardial tissue occurs. Thrombocytopenia, encephalitis, and necrosis of testis are also occasionally noted. Prominent pitted scarring is likely due to the destruction of sebaceous glands (6).
The exact cause of death due to smallpox is not completely understood. Secondary bacterial infections have been posited to play a role in death, but recent data do not support their role in fatalities. One retrospective study attributed many deaths to cytotoxicity or immune complex disease (7).
CLINICAL DISEASE AND EPIDEMIOLOGY
Presentation
Clinical presentations of smallpox can vary depending on the patient’s vaccination status, level of nutrition, and infectious strain, among a host of unknown factors. Smallpox illness has three phases: incubation, prodrome, and rash. Infection occurs via the respiratory mucosa, and an incubation period of 10 to 14 days on average occurs before a prodromal period of 2 to 4 days. The prodrome is characterized by fever, malaise, vomiting, headache, backache, and myalgia. The prodromal phase can be severe enough to confine many patients to bed and has been described to resemble a severe influenza illness (2,8).
Rash initially presents as an enanthem on the mucous membranes of the mouth, tongue, and oropharynx; within 24 hours, a rash, ultimately with centrifugal distribution, develops. Usually, the rash is first evident on the face, then proximal extremities, distal extremities (including palms and soles), and trunk (Fig. 104-1). The rash develops from macules to papules to vesicles to pustules over the course of 1 to 10 days postprodrome (Fig. 104-2). Lesions on any one part of the body generally present in the same stage of development during the course of the rash, and lesions present with a centrifugal density. Lesions are deep, firm, and become umbilicated (Fig. 104-3). Lesion numbers can be denser in areas of trauma or inflammation—the “garter effect”—and are noted in areas where there are scratches or irritation. Scabs eventually form and fall off approximately 2 to 3 weeks postinitial rash onset and leave pronounced deep scars and hypo-and/or hyperpigmentation (2,6,8). Nonfatal severe complications associated with variola infections include panophthalmitis, blindness, keratitis, corneal ulcers, osteomyelitis, arthritis, orchitis, and encephalitis.
Forms of Variola
Smallpox has been categorized in a number of different ways including variola major and minor, based on epidemiological criteria such as case fatality rates. Variola major and variola minor, on average, have case fatalities of about 30% and ≤1%, respectively. Variola-alastrim strains (which caused disease in Brazil in the 20th century) have been discretely biologically and genetically discriminated from variola major. However, certain African variola minor isolates by laboratory assays are more like variola major than alastrim (9). Surveillance data (which may be biased by health-seeking behaviors) suggest that nearly 90% of patients develop variola major. Variola major was categorized by the WHO into eight clinical types. Three types of “ordinary” disease are discriminated by the density of rash presentation on the face and body: ordinary discrete, ordinary semiconfluent, and ordinary confluent. In hospitalized patients, these forms are characterized to have mortality rates of 30%, 37%, and 62%, respectively, in unvaccinated individuals and mortality rates of 3%, 8%, and 26%, respectively, in vaccinated individuals. The terms discrete, semiconfluent, and confluent refer to the density of the lesions (6,10).
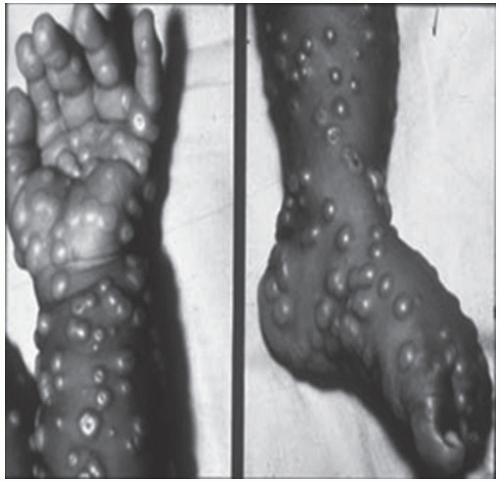 FIGURE 104-1 Smallpox: lesions on palms and soles. (From World Health Organization.) (See color insert.) |
Less severe, somewhat atypical disease, described as modified smallpox, exhibits a faster disease time course with more superficial lesions when compared to ordinary forms of variola major. Another less pathogenic form, variola sine eruption, is characterized by a febrile illness without rash and little to no viral transmission. Modified smallpox and variola sine eruption occur mainly in vaccinated individuals with little associated mortality (2,6).
The most severe, and rare, manifestations of disease (<3-5% of hospitalized patients) are flat and hemorrhagic forms of smallpox. Flat-type smallpox describes a disease in which the lesions appear flat, likely because of significant tissue edema. In hospitalized patients, the mortality rate is approximately 97% in unvaccinated and 67% in vaccinated individuals. Hemorrhagic smallpox is characterized to have two variants: early and late. In early disease, the characteristic discrete, raised pustules seen in ordinary smallpox do not develop. Instead painful, erythematous,
petechial lesions appear. In late hemorrhagic disease, some characteristic lesions develop, and hemorrhage is noted at the base of the lesions. The incubation period for early hemorrhagic smallpox is relatively short and death occurs within 5 or 6 days of rash onset. Death, regardless of vaccination status, occurs in approximately 95% of hospitalized individuals and is most likely due to massive mucosal hemorrhage (2,6,10).
petechial lesions appear. In late hemorrhagic disease, some characteristic lesions develop, and hemorrhage is noted at the base of the lesions. The incubation period for early hemorrhagic smallpox is relatively short and death occurs within 5 or 6 days of rash onset. Death, regardless of vaccination status, occurs in approximately 95% of hospitalized individuals and is most likely due to massive mucosal hemorrhage (2,6,10).
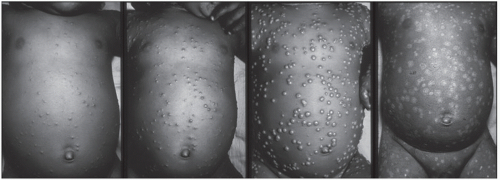 FIGURE 104-2 Smallpox: progression of lesions on the abdomen of child days 4, 5, 8/9, and 20. (From World Health Organization.) (See color insert.) |
 FIGURE 104-3 Firm, deep-seated pustular lesions on right arm (top) and umbilicated lesions on the leg of a 1-year-old (bottom). (Top: From CDC/Dr. John Noble Jr. Bottom: From CDC/Dr. Robinson.) (See color insert.) |
Diagnosis
There are a number of illnesses that can be misdiagnosed as smallpox. These have included, and continue to include, varicella (chickenpox), monkeypox, disseminated herpes zoster and herpes simplex, impetigo, drug-induced rashes, erythema multiforme, Stevens-Johnson syndrome, scabies, molluscum contagiosum, and enteroviral infections, especially hand, foot, and mouth disease. The disease most commonly mistaken for smallpox during and after eradication is varicella. Clinical features of varicella that distinguish it from smallpox are a short prodromal phase lasting 1 to 2 days, fever with onset of rash, centripetal rash distribution, lesions in varying stages of development, and shortened lesion progression from vesicles to crusting (3). Human monkeypox, a zoonotic disease endemic to central and western Africa, resembles smallpox in appearance, but patients will typically experience lymphadenopathy as part of their clinical course. Human monkeypox has not been seen in the United States since the 2003 outbreak related to importation of African rodents (11).
Many medical professionals have no experience with smallpox; therefore, an algorithm was developed by CDC that separates patients into three risk categories for smallpox —high, moderate, or low (Fig. 104-4) (3). This algorithm (a) provides information about the symptoms of smallpox and other causes of febrile, vesicular/pustular rash illnesses likely to be confused with smallpox and (b) limits laboratory testing to high-risk patients reducing the likelihood of false-positive test results (12). The algorithm can be found at http://emergency.cdc.gov/agent/smallpox/diagnosis/evalposter.asp.
Because of bioterrorism concerns for the potential malevolent use of variola, screening for variola virus from specimens of high-risk individuals can be performed in the U.S. at specific Laboratory Response Network (LRN) reference laboratories. Absent circulating disease, if screening at an LRN facility is positive for variola virus, more extensive testing and confirmation is performed at CDC before results are released. Real-time polymerase chain reaction is the gold standard for detection of variola virus. Previously, unique viral growth on chorioallantoic membrane
provided definitive confirmation. Other laboratory testing diagnostics such as enzyme-linked immunosorbent assay, electron microscopy, and immunohistochemical staining can diagnose a poxvirus infection; however, these tests are not specific for variola virus. With the exception of parapoxviruses, poxviruses have identical morphology by electron microscopy making it impossible to differentiate one species from another. Assays relying on immune reagents will, at least, identify all members of a poxvirus genus; orthopoxviruses share >90% genetic similarity causing antibodies to cross-react among member species (3).
provided definitive confirmation. Other laboratory testing diagnostics such as enzyme-linked immunosorbent assay, electron microscopy, and immunohistochemical staining can diagnose a poxvirus infection; however, these tests are not specific for variola virus. With the exception of parapoxviruses, poxviruses have identical morphology by electron microscopy making it impossible to differentiate one species from another. Assays relying on immune reagents will, at least, identify all members of a poxvirus genus; orthopoxviruses share >90% genetic similarity causing antibodies to cross-react among member species (3).
 FIGURE 104-4 Evaluating patients for smallpox: acute, generalized vesicular, or pustular rash illness protocol. (From Centers for Disease Control and Prevention. Acute, generalized vesicular or pustular rash illness testing protocol in the United States. Available at http://emergency.cdc.gov/agent/smallpox/diagnosis/pdf/poxalgorithm11-14-07.pdf. Accessed June 1, 2011.) (See color insert.) |
Therapy
Currently, no antivirals are licensed for the treatment of any orthopoxvirus infection. There are active research programs evaluating various compounds against orthopoxviruses, including variola, in in vitro tissue culture, and in various orthopoxvirus-challenge animal disease model systems. Supportive therapy should be offered to smallpox patients. The time period between exposure and case identification or symptom onset is a critical factor in consideration for the use of postexposure prophylaxis (in the form of vaccination) or the use of investigational antiviral medications (2,4,13).
Patterns of Transmission
The infectiousness of a smallpox case depends upon the amount of viral shedding in oropharyngeal secretions and the number and distance of face-to-face contacts with susceptible persons. Smallpox patients have maximum infectivity during the first week of rash when large amounts of virus are being shed from the mouth and pharynx. Severe cases of smallpox typically shed larger amounts of oropharyngeal virus than those with modified-type smallpox. Although a large amount of virus can be detected in smallpox scabs, their infectivity is considerably less due to the enclosure of viral particles within hard dry scabs (4). Transmission rarely occurs before the first day of rash (14). Epidemiologic studies have found that most cases of secondary smallpox caused by importation from an endemic area occurred within 3 weeks of initial exposure (15).
The most frequently infected group is the household or family because of the significance of face-to-face contact in transmission. The secondary attack rate of variola major has
ranged from 1.2% to 88% in close contacts and is significantly affected by the vaccination status of the contact. Because patients with variola major in the prodromal phase usually fall quite ill, they separate themselves from the community, but not from their household contacts. The average attack rate for unvaccinated family contacts was 58.4%, and 3.8% in vaccinated contacts. On the other hand, cases infected with variola minor are more mobile causing more disease within the community despite less viral shedding (4).
ranged from 1.2% to 88% in close contacts and is significantly affected by the vaccination status of the contact. Because patients with variola major in the prodromal phase usually fall quite ill, they separate themselves from the community, but not from their household contacts. The average attack rate for unvaccinated family contacts was 58.4%, and 3.8% in vaccinated contacts. On the other hand, cases infected with variola minor are more mobile causing more disease within the community despite less viral shedding (4).
Smallpox shows a seasonal variation in incidence with a predilection for winter and spring. However, seasonal fluctuation is limited in areas with uniform temperature and humidity. Aside from the environmental factors that may prolong the viability of virus, cool temperature and low humidity, other considerations have been given to the seasonal incidence of smallpox. These include (a) changes in mucous membrane permeability, (b) alterations in resistance because of changes in diet, and (c) the effect of climate on social activities (4).
VACCINATION
Edward Jenner demonstrated the principles of vaccination in 1796 when he used material from human cowpox lesions to protect individuals against smallpox. Today, live vaccinia virus is used to vaccinate against smallpox. Vaccinia virus is a closely related, yet distinct Orthopoxvirus species from variola. Routine vaccination was common in many countries until the early 1970s (4).
Variola virus has no nonhuman animal reservoir, and infection can be prevented with a single-dose, recent vaccination: these two characteristics made eradication a feasible accomplishment. WHO began an intensive surveillance, containment, and eradication campaign in 1967. Surveillance and containment consisted of the following five steps: (a) identification of cases, (b) isolation of patients, (c) identification of ring or close contacts, (d) vaccination of the ring contacts, and (e) vaccination of the associates of the ring contacts. This approach was used to eradicate smallpox and is recommended by WHO for use today if smallpox reappears (2,4). However, if a reintroduction occurs, widescale vaccination may occur in several countries.
 FIGURE 104-5 Vaccination site progression sequence in a normal primary vaccinee days 4, 7, 14, and 21. (From Centers for Disease Control and Prevention.) (See color insert.) |
In late 2007, the Food and Drug Administration licensed a new smallpox vaccine to replace Dryvax®. This new vaccine, ACAM2000®, is a cell culture grown, fully replicative vaccinia virus derived from a clonal isolate of Dryvax®. ACAM2000® was chosen based on its similar efficacy to Dryvax® (16). Focus of current research on smallpox vaccines is the further development of replication competent cell culture-derived vaccinia; replication deficient, highly attenuated vaccinia; and DNA-or protein-based vaccines. Preclinical and clinical trials are currently underway for some of these newer vaccines to determine their efficacy (17).
The preferred site for vaccination is the upper arm over the deltoid muscle. Vaccine is delivered by scarification using a sterile bifurcated needle that has been dipped into the rehydrated suspension. Fifteen perpendicular strokes to the skin are given through the droplet within a diameter of about 5 mm. The appearance of a drop of blood indicates that the strokes were vigorous enough to puncture the skin. Following successful primary vaccination, a major cutaneous reaction should appear at the site of inoculation by day 6 to 8 (Fig. 104-5). Within 2 to 5 days postvaccination, a papule will appear that will become vesicular, then pustular, and reach maximum size at day 8 to 10. The pustule will dry forming a scab which typically separates within 14 to 21 days, often leaving a pitted scar, and hypo- or hyperpigmentation is not unexpected. Persons who are revaccinated after successful primary vaccination may have a modified cutaneous response but this does not necessarily indicate an unsuccessful vaccination (18).
Because the current smallpox vaccine is a live, fully replicative vaccinia virus and can cause secondary transmission,
the inoculation site must remain covered until the scab has completely detached and a new epidermal layer has formed. A gauze bandage held in place by adhesive tape should loosely cover the site. If the vaccinee has an occupation that puts him or her in direct contact with patients, the gauze should be covered with an additional barrier using a semipermeable dressing and a layer of clothing. The use of only a semipermeable dressing is not recommended as it might lead to maceration of the vaccination site. Maceration can cause prolonged irritation and itching, potentially leading to increased touching or scratching and therefore contamination of hands. If maceration does occur, the lesion should be left uncovered to allow the site to dry. This is only suitable when a healthcare worker has no direct contact with patients or other persons. The vaccination site must remain covered until the scab separates (19). Contaminated bandages should be sealed in a plastic bag and thrown in the trash. After direct contact with the vaccination site, hands should be washed with soap and warm water or with alcoholbased hand rubs. Potentially contaminated clothes, towels, or sheets should be washed separately in warm water (18).
the inoculation site must remain covered until the scab has completely detached and a new epidermal layer has formed. A gauze bandage held in place by adhesive tape should loosely cover the site. If the vaccinee has an occupation that puts him or her in direct contact with patients, the gauze should be covered with an additional barrier using a semipermeable dressing and a layer of clothing. The use of only a semipermeable dressing is not recommended as it might lead to maceration of the vaccination site. Maceration can cause prolonged irritation and itching, potentially leading to increased touching or scratching and therefore contamination of hands. If maceration does occur, the lesion should be left uncovered to allow the site to dry. This is only suitable when a healthcare worker has no direct contact with patients or other persons. The vaccination site must remain covered until the scab separates (19). Contaminated bandages should be sealed in a plastic bag and thrown in the trash. After direct contact with the vaccination site, hands should be washed with soap and warm water or with alcoholbased hand rubs. Potentially contaminated clothes, towels, or sheets should be washed separately in warm water (18).
The Advisory Committee on Immunization Practices lists a number of contraindications to smallpox vaccination in a routine nonemergency setting for both vaccinees and their household contacts. For both, these include a past or present history of eczema or atopic dermatitis; acute, chronic, or exfoliative skin conditions such as burns, chickenpox, or Darier’s disease; immunodeficiency or those currently on immunosuppressive therapy; inflammatory eye diseases leading to use of steroid eye drops; and pregnancy or plans to become pregnant in the next 4 weeks. Additional contraindications for vaccinees only include: allergy to smallpox vaccine components, including polymyxin B, neomycin, streptomycin, and phenol; symptomatic or asymptomatic heart disease or three or more cardiac risk factors (hypertension, diabetes, hypercholesterolemia, heart disease at 50 years of age in a first degree relative, or current smoker); breastfeeding; persons under 18 years of age, especially infants <12 months, and those older than 65 years; severe allergy to latex; or “moderate” to “severe” illness at vaccination time. There are no absolute contraindications to vaccination for a person with a high-risk exposure to smallpox (18,20,21).
In the United States, routine smallpox vaccination of civilians was discontinued in 1971 following reduction of smallpox importations in the 1960s. In 1976, routine vaccination of healthcare workers was also discontinued (22). In 2002, a preparedness plan was initiated to protect the United States against a possible smallpox bioterrorist attack. This plan calls for vaccination of both military and civilian personnel. Under the U.S. Civilian Smallpox Preparedness and Response Program, groups of public health and medical response teams who would care for smallpox patients during the first 7 to 10 days of an outbreak were voluntarily vaccinated (Table 104-1). Approximately 40,000 civilian personnel received licensed vaccine from January to December 2003 (23). Since 2003, CDC continues to provide smallpox vaccine to state public health authorities for vaccination of smallpox response team members. CDC recommends revaccination of volunteer responders on an “out-the-door” basis, meaning only after a smallpox outbreak has been confirmed or is highly suspected, or there is credible evidence of a release or imminent release (24). The US military continues to vaccinate personnel who serve in high-risk parts of the world. Additionally, laboratory researchers who work with or may be exposed to nonhighly attenuated vaccinia virus are often vaccinated (21,25,26).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


