The foetoplacental unit
The placenta produces several proteins, including hCG and (human) placental lactogen. It also produces large amounts of steroid hormones and is the main source of progesterone during pregnancy.
Human chorionic gonadotrophin
There are several pregnancy-specific proteins, all of which normally originate in the trophoblast. The most commonly measured is hCG. Following synthesis, hCG is secreted into the maternal circulation. There is a surge in maternal [hCG] in early pregnancy, peak blood levels being reached at 12 weeks; thereafter, production of hCG rapidly declines. hCG becomes detectable in urine about 10 days after conception, and this forms the basis of readily available pregnancy tests.
Trophoblastic tumours secrete hCG. These tumours can occur in males and females, and they include hydatidiform mole and choriocarcinoma, both of which may secrete hCG in very large amounts. A female who is found to be excreting hCG, and who is not pregnant, most frequently has a tumour of the trophoblast; in males, testicular teratoma is the most common source (p. 250).
Steroids in pregnancy
Oestrogens and progesterone are secreted by the corpus luteum during the first 6 weeks of pregnancy, but after this the placenta is the most important source of these steroids. Oestriol is the oestrogen produced in the greatest amounts, but oestradiol-17β and oestrone are also produced in large amounts. The placenta cannot synthesise oestriol de novo, but it can produce oestriol from C-19 adrenal steroids that are supplied by the foetal adrenal in the form of DHEAS. The oestriol produced in this way is secreted into the maternal and foetal circulation. Oestriol production thus requires the involvement of both the placenta and the foetus, and recognition of this interdependence led to the concept of the foetoplacental unit.
Effect of pregnancy on biochemical tests
Reproductive hormones
Serum [prolactin], [oestrogens] and [testosterone] show a steady increase in pregnancy, as does the concentration of SHBG. The concentrations of growth hormone and the pituitary gonadotrophins are decreased. However, some less specific methods for the measurement of LH may show cross-reaction with hCG, leading to apparent high LH levels.
Cortisol
There are large increases in serum [cortisol] due to increased serum [CBG], but the diurnal rhythm is retained. However, increased free and total cortisol levels in pregnancy may also be related to resetting of the sensitivity of the HPA axis and not merely to raised levels of CBG, progesterone or CRH. There is also an increase in serum [free cortisol] and in the 24-h urinary excretion of cortisol. This may be related to a resetting of the HPA axis and also the production of an ACTH-like substance by the placenta that is not completely suppressible by low- or high-dose glucocorticoids such as dexamethasone. This may help to explain why pregnant women often show intolerance of glucose and occasionally develop Cushingoid features. These changes make the diagnosis of Cushing’s syndrome difficult in pregnancy, and several variations in the work-up, when compared with non-pregnant women, may be required. An absence of diurnal variation is a useful clue to the diagnosis.
Thyroid function tests
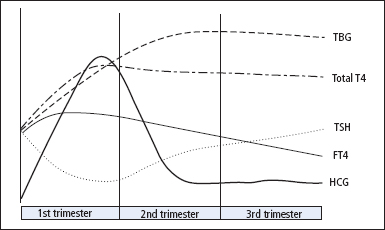
During pregnancy, oestrogen production increases and TBG concentrations rise, leading to an increase in total T4 and total T3. There is also a large increase in the concentration of hCG, a hormone that has a mild stimulatory effect on thyroid hormone production. As a consequence, [FT4] and [FT3] may increase slightly during the early part of the first trimester, which, through the normal negative feedback loop, leads to a fall in serum TSH sometimes to undetectable concentrations. In the second and third trimesters, the serum [FT4] and [FT3] decrease and may fall below the reference range derived from non-pregnant women (Figure 11.1). The magnitude of this fall in free thyroid hormones is method dependent. After delivery, levels of thyroid hormones and TSH normally return to the pre-pregnant state. Trimester-related reference ranges should be applied for TSH and thyroid hormones if these are available; for free hormones, these reference ranges are also method dependent.
Figure 11.1 Changes in TSH, thyroid hormones, hCG and TBG in normal pregnancy. For TSH and thyroid hormones it is important to use gestational or trimester-related reference ranges. In some pregnancies TSH may fall to <0.1 mU/L in the first trimester. Total T3 and FT3 follow the same pattern as total T4 and FT4, respectively.
Plasma volume and renal function
During pregnancy, the plasma volume and GFR increase, sometimes by as much as 50%. This is accompanied by decreases in, for example, serum [sodium], [urea] and [creatinine].
Serum lipids and proteins
Serum [triglyceride] may increase as much as 3-fold in pregnancy; serum [cholesterol], LDL and HDL increase to a lesser extent. Serum [albumin] and [pre-albumin] fall because of the increase in plasma volume. Plasma [fibrinogen] and [ceruloplasmin] increase.
Liver function tests
In pregnancy, the placental isoenzyme of ALP is released, and total ALP activity in serum may rise to as much as three times non-pregnant levels. In contrast, the expansion of the extracellular fluid leads to a fall (~20%) in the activities of the transaminases and GGT and in the concentration of bilirubin.
Iron and ferritin
During pregnancy, increased maternal red cell synthesis and transfer of iron to the developing foetus cause a greater demand for iron. Unless iron supplements are given, iron stores generally fall, with accompanying falls in serum [ferritin] and plasma [iron], and rises in serum [transferrin] and total iron binding capacity (TIBC).
Complications in pregnancy
Ectopic pregnancy
In ectopic pregnancy, serum [hCG] fails to rise at the normal rate (approximately doubling every 2–3 days). If levels have failed to rise by 66% in 2 days, there is a 90% chance of an abnormal pregnancy. In practice, the diagnosis is made on a high index of clinical suspicion, qualitative pregnancy tests, ultrasound and, if indicated, laparoscopy.
Diabetes mellitus
Women with type 1 diabetes are at greater risk from both diabetic and obstetric complications during pregnancy. Rates of foetal and neonatal complications including late intrauterine death, foetal distress, congenital malformation, hypoglycaemia, respiratory distress syndrome and jaundice are also increased. To minimise these risks, it is essential that maternal glucose control and HbA1c are optimised prior to conception and that tight control is maintained throughout pregnancy. Particular emphasis is placed on the need for careful home glucose monitoring (4–6 times a day) and intensive insulin regimens. Women should aim to maintain blood glucose and HbA1c concentrations as near to the non-diabetic range as possible without excessive risk of hypoglycaemia. Type 2 diabetes is less common during the reproductive years, but its management during pregnancy should follow the same intensive pattern.
‘Gestational diabetes mellitus’ is the term used to describe the abnormal glucose tolerance or diabetes mellitus that may develop during pregnancy. It is particularly important to identify women with undiagnosed type 1 or type 2 diabetes mellitus as urgent action is required to normalise metabolism. The diagnosis of gestational diabetes mellitus is made on the basis of an OGTT. Glucosuria detected at routine antenatal testing may suggest the need for an OGTT, but may have no significance, since the renal threshold for glucose tends to be lowered in pregnancy. One approach is to screen women with appropriate risk factors, such as a family history of diabetes mellitus, or a previous large baby. Also, glucosuria is more significant if detected on the second specimen of urine passed after an overnight fast (i.e. the first specimen passed is discarded). Mild abnormalities should be reassessed not less than 6 weeks after delivery. In the majority of cases of gestational diabetes, the response to a GTT reverts to normal after the pregnancy, but about 50% of patients go on to develop diabetes mellitus within the next 7 years.
Thyroid disorders
Screening for thyroid disorders in pregnancy
The following categories of patient should have TSH and FT4 measured preferably prior to conception or, if not, at booking:
- Current thyroid disease
- Previous history of thyroid disease
- Family history of thyroid disease
- Goitre or other features of thyroid disease
- Type 1 and type 2 diabetes.
Management of thyroid disease requires close liaison between the GP, endocrinologist, obstetrician and community midwife.
Hypothyroidism
The developing foetal brain requires optimal thyroxine levels from early in the first trimester of pregnancy. The foetus relies on maternal thyroxine until 12 weeks gestation, when its own thyroid gland develops. Overt untreated hypothyroidism is associated with foetal loss, gestational hypertension, placental abruption, poor perinatal outcome and severe neurodevelopmental delay. The offspring of women with subclinical hypothyroidism, whose FT4 levels are in the lowest 10% of the reference range in the first trimester of pregnancy, have significant neurodevelopmental delays at the age of 2 years.
There is an increased requirement for T4 in pregnancy, and mothers with hypothyroidism are required to have the dose of T4 increased by 25–50 mg/day when pregnancy occurs. It is thus very important to ensure adequate thyroxine replacement from as early as 5 weeks gestation.
Assessing hypothyroidism in pregnancy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


