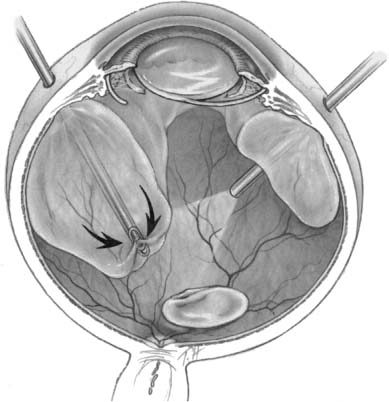
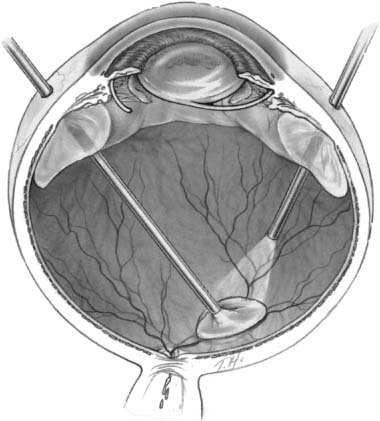
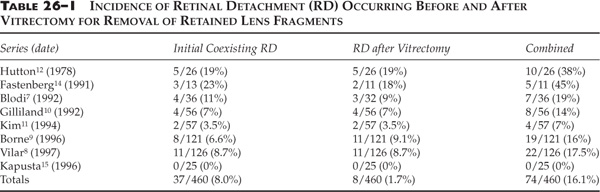
Chapter 26 Cataract surgery is the most common operation performed in the United States and has one of the highest success rates.1 Phacoemulsification techniques have revolutionized the field of cataract surgery, and have allowed for the development of innovations such as small incisions and foldable intraocular lens implants. More recently, sutureless corneal incision techniques have come into favor, and many cataract operations are done with topical anesthesia, thus eliminating retrobulbar and peribulbar anesthesia and the associated risks. These remarkable advances in technology have led to equally remarkable refinements in the surgical skills of the ophthalmologist, operations of shorter duration and higher surgical case loads. Expectations of patients have dramatically increased as a result of the rapid visual and physical rehabilitation of what has become a relatively minor operation. Commercial advertising for cataract surgery has fueled this increase in expectations by minimizing the need for injections or patches, reducing postoperative pain, and emphasizing that return to normal activities may be possible within days. The uncomplicated cataract operation with implant is indeed a minor operation with remarkable outcomes in the vast majority of cases. Although the complication rate is quite low, the consequences can cause significant visual loss. Awareness of these complications and appropriate management can minimize the visual impairment and enhance the outcome and recovery for the patient, thereby reducing the risk of litigation that so often arises when patient expectation is not met. The posterior segment complications of cataract surgery are not specific to phacoemulsification. This chapter reviews the important clinical features and treatment options, with emphasis on phacoemulsification. Posterior segment complications include retained lens fragments, dislocated intraocular lens implant, endophthalmitis, suprachoroidal hemorrhage, and needle penetration of the globe for anesthesia. The most common clinical situation leading to retained lens fragments in the vitreous is posterior capsular rupture, with loss of lens fragments posteriorly into the vitreous cavity during the fragmentation phase of phacoemulsification. The displaced lens fragment may involve the entire nucleus or any fraction of it. The best estimate of the incidence of posteriorly displaced lens fragments is 0.3%.2 Once posterior capsule rupture occurs, the surgeon must proceed with extreme caution in using a limbal approach to retrieve displaced lens fragments. Although in some instances converting to a larger incision and using a lens loop or forceps will allow retrieval of a nuclear fragment before it migrates posterior to the capsule, once the fragment falls posteriorly a high chance of further complication ensues with limbal retrieval attempts. Some surgeons advocate vigorous attempts at retrieving the lost lens nucleus from the limbal cataract incision by probing posteriorly with a lens loop or other instrumentation, or by using high volumes of infusion fluid to create vortex currents to float the lens fragment anteriorly.3 However, vigorous attempts at retrieving a posteriorly dislocated nucleus from the limbus with high volumes of intraocular fluid or posterior manipulation of the instrument has been associated with giant retinal tears that have a poor prognosis for visual acuity.4 In a recent case managed by one of the authors, the nucleus was found underneath a giant tear retinal detachment in the inferior nasal quadrant. Posterior loss of lens fragments is usually recognized intraoperatively after posterior capsular rupture. Occasionally retained lens material may present with chronic intraocular inflammation and no visible fragments in the posterior pole. The degree of intraocular inflammation usually reflects the size of the retained lens fragment, the time interval since cataract surgery, individual inflammatory reactivity, and the extent of previous intraocular manipulations. Associated clinical signs including corneal edema, glaucoma, uveitis, and vitreous opacities. Initially these findings are frequently mild, especially in the immediate postoperative interval, but, over time, may worsen and lead to other complications such as retinal detachment, causing profound visual loss.5,6 The size of the lens fragment and severity of intraocular inflammation usually forms the basis for surgical intervention. Operations should probably be performed if the lens nucleus fragment is larger than about 3 mm in diameter because secondary inflammatory complications will virtually always ensue. Eyes with very small retained fragments have a better prognosis and can often be observed indefinitely. However, if inflammation has not subsided by 1 to 2 weeks, surgical intervention should be considered regardless of how small the retained fragment is, because other occult fragments may be harbored behind the iris. Chronic glaucoma was reported to be more common when the subsequent vitrectomy was performed more than 3 weeks following surgery.7 Other studies have not found any outcome differences between earlier and later intervention.8–11 A study of 126 patients before vitrectomy for posterior chamber lens fragments after phacoemulsification demonstrated a 37% prevalence of intraocular pressure (IOP) above 30 mm Hg preoperatively.8 The IOP normalized in all except 3% of patients after vitrectomy. The patient’s overall clinical situation may influence timing, but usually surgery to remove retained lens material is performed within 2 weeks of the original cataract surgery to expedite visual rehabilitation, to break the cycle of progressive lens-induced inflammation, and to lessen risks of secondary lens induced glaucoma. These goals may be logistically maximized when lens fragment retrieval and removal can be expertly performed during the original cataract operation. When this is not feasible, a delay of several days or even weeks may be equally effective, because the inflammation, corneal edema, and glaucoma will improve with topical treatment over several days following cataract surgery. A variety of techniques have been described for use by the anterior segment surgeon at the time of lens fragment loss. Although some cases may be satisfactorily managed through a limbal incision, pars plana vitrectomy techniques probably offer superior results in most cases.7–15 There are three basic surgical techniques for lens fragment removal using a pars plana vitrectomy: (1) ultrasonic fragmentation, (2) mechanical crushing of the nucleus between two instruments, and (3) limbal extraction of hard nuclear fragments. The availability of modern phacofragmentation units generally precludes the need for the latter two techniques, but they are options when fragmentation is unavailable or in cases with an extremely hard nuclear fragment. There are three key elements in successful lens fragment removal technique. First, adequate initial vitrectomy avoids unintended vitreous traction during phacofragmentation (Fig. 26–1). Second, reducing fragmentation power to only 5 to 10% of maximum allows more efficient nuclear extraction by continuous occlusion of the suction port minimizing the risk of mechanical retinal trauma from projectile fragments. This maneuver also minimizes the chance of fragments dropping back onto the retina even though these fragments rarely strike the retina with sufficient force to damage the retina. Third, fragments should be cautiously aspirated from the retinal surface and moved to the mid-vitreous before activating ultrasonic fragmentation to avoid suction or ultrasonic damage to the retina (Fig. 26–2). Perfluorocarbon liquids have been described to float the nucleus anteriorly to facilitate removal,16,17 but are most useful when retinal detachment coexists.18 Techniques for reattaching the retina when associated with retained lens fragments are similar to those for other complex retinal detachments.19,20 Most anterior segment surgeons proceed to intraocular lens (IOL) insertion at the time of the original cataract surgery, as is recommended by the majority of vitreous surgeons in spite of the intraoperative complication of posteriorly displaced lens fragments. If there is sufficient capsular support, the IOL is placed in the posterior chamber (PC). If not, suture fixation techniques may be used 21–32 or an anterior chamber (AC) IOL may be placed. If the cataract surgeon is reluctant to insert the IOL primarily, it can be inserted at the time of the subsequent vitrectomy. FIGURE 26–1 Attention to complete central vitrectomy allows access to retained lens fragments. (From Regillo CD, Brown GC, Flynn HW Jr. Vitreoretinal Disease: The Essentials. New York: Thieme; 1999:568, Fig. 35–5.) FIGURE 26–2 Low ultrasonic fragmentation power allows more controlled removal of fragments. (From Regillo CD, Brown GC, Flynn HW Jr. Vitreoretinal Disease: The Essentials. New York: Thieme; 1999:569, Fig. 35–6.) The visual results of managing such cases are generally good.7–16 Postoperative visual acuity of 20/40 has been achieved in the majority of patients in many series. One series reported only a 41% rate of 20/60,7 but recent series report 60 to 82% rates of 20/40.8–15 This apparent improvement may reflect changing patterns of cataract surgery technique because a poorer prognosis has been suggested with dropped lens nuclei when extracapsular cataract extraction (ECCE) techniques are used compared to phacoemulsification extraction methods.7 Postoperative complications related to vitrectomy surgery may be difficult to differentiate from those attributable to complicated cataract surgery, and may include corneal edema, glaucoma, persistent intraocular inflammation, and new retinal detachment.33 Retinal detachment coexisted with retained lens material in 8.0% of reported series and retinal detachment has been reported after vitrectomy for removal of retained lens fragments in 8.3% of reported series (Table 26–1). Thus, it is of critical importance to evaluate the retina throughout the perioperative course in such patients. One author suggested prophylactic scleral buckle in cases undergoing vitrectomy for retained lens fragments,34 but this entails needless surgery (and its incumbent complications) in 90% of eyes. Recommendations to the anterior segment surgeon experiencing the complication of posterior dislocation of lens fragments include (1) avoid loss of fragments by immediate use of dispersive viscoelastic to trap nuclear fragments; (2) use a Sheets’ glide to stabilize the nucleus in the presence of a large capsular tear; (3) attempt lens fragment retrieval only if fragment is readily accessible; (4) perform anterior vitrectomy as necessary to avoid anterior vitreous prolapse into the surgical incision; (5) if possible, insert PC IOL using residual posterior capsule or AC IOL, as merited by the situation; (6) close the cataract wound in a standard fashion and remove viscoelastic (sutures are indicated to ensure wound integrity during subsequent vitrectomy); (7) prescribe frequent postoperative topical antiinflammatory treatment and IOP-reducing agents; and (8) refer the patient for vitreoretinal consultation within a few days for initial evaluation (Table 26–2). If the opportunity and expertise exists, perform the vitrectomy and retrieval of displaced lens fragments at the same operation. Recommendations for the vitreoretinal surgeon include (1) initially observe eyes with minimal inflammation and a very small lens fragment; (2) continue or initiate treatment with topical corticosteroids and antiglaucoma agents; (3) intervene surgically if inflammation or IOP is not controlled, or if the fragment is estimated to measure ≥3 mm; (4) delay surgery as necessary to allow for initial treatment of postoperative inflammation and allow clearing of corneal edema; (5) perform adequate core vitrectomy before attempting phacofragmentation; (6) use low fragmentation power settings (5 to 10%) for more efficient removal of small fragments; (7) be prepared for secondary IOL insertion in aphakic eyes or IOL exchange in some pseudophakic eyes; and (8) examine peripheral retina for possible retinal tear or detachment (Table 26–3).
POSTERIOR SEGMENT
COMPLICATIONS
RETAINED LENS FRAGMENTS
CLINICAL FEATURES
SURGICAL INDICATIONS
SURGICAL TECHNIQUES
OUTCOMES OF VITRECTOMY FOR RETAINED LENS FRAGMENTS
RECOMMENDATIONS FOR MANAGEMENT OF RETAINED LENS FRAGMENTS
Attempt retrieval only if easily accessible Do anterior vitrectomy to avoid vitreous prolapse Insert intraocular lens (IOL) as safe and indicated Do standard wound closure and viscoelastic removal Use frequent topical postoperative antiinflammatory and intraocular pressure (IOP)-lowering agents Request vitreoretinal consultation promptly |
INTRAOCULAR LENS DISLOCATION
Postoperative decentration of PC IOLs occurs in 0.2 to 1.2% of cases and usually does not require treatment.35,36 A less common but more significant complication is IOL dislocation into the vitreous cavity. A common element in all cases is insufficient posterior capsule support. This is typically due to posterior capsular rupture during cataract extraction.When dislocation occurs within the first few days or weeks after surgery, the cause may be less apparent and may be the result of unknowingly placing the IOL through a posterior capsular defect onto the anterior hyaloid, or as a result of subsequent IOL haptic rotation out of a zone of residual capsule remnants. Late dislocation is less common and may be due to traumatic37 or spontaneous loss of zonular support such as in eyes with pseudoexfoliation syndrome.
CLINICAL CHARACTERISTICS
Complete dislocation of PC IOLs into the vitreous cavity is typically observed within the first week following surgery (26 of 32 cases in one series38). Less commonly it occurs during surgery or several months after surgery. The presenting visual acuity with aphakic correction may be very good, but is commonly decreased to a moderate degree despite the best spectacle correction. Patients with luxated or subluxated PC IOLs are usually symptomatic because of the variable position of the optic in the visual axis. In addition, a mobile PC IOL may also generate unique floater-like symptoms, or even lead to pupillary block glaucoma.
Observe initially on topical agents Assess need for surgical intervention Optimize timing Complete vitrectomy before fragmentation Use ultrasonic fragmentation at low power Insert IOL as indicated Carefully examine retinal periphery |
The presenting symptoms of patients with dislocated IOLs range from minimally symptomatic lens decentration to complete luxation into the vitreous cavity.36 Decentration usually refers to mild malposition with the optic still covering more than half of the pupillary space. In many cases of decentration one haptic is in the ciliary sulcus and the other is in the capsular bag. Progressive decentration may become apparent with progressive capsular fibrosis. Patients at this, the milder end of the spectrum, usually present several weeks after cataract extraction with good visual acuity, normal IOP, and without inflammation. Visual symptoms usually are mild and may be related to glare from the edge of the optic.
MANAGEMENT OPTIONS
There are four general classes of management options for dislocated IOLs: observation, removal, exchange, or repositioning.38–45 The management plan and timing are formulated based on clinical factors such as the type of IOL and any observed secondary complications.
Patients presenting with substantial intraocular inflammation, retinal detachment, or with cystoid macular edema (CME), especially when associated with vitreous to the cataract incision, constitute definite candidates for surgery. Although a completely dislocated IOL may be well tolerated in many patients, the difficulty in visual rehabilitation necessitates surgical intervention in most. For symptomatically subluxated IOLs, surgery may be performed via a limbal or a pars plana approach. Patients with less extensive subluxation can be managed through a limbal incision with minimal or no anterior vitrectomy if the posterior capsule is largely intact. However, if there is a large posterior capsular rent, vitrectomy using a pars plana approach may offer optimal control to achieve the goals of surgery and address unforeseeable intraoperative complications.
Observation
IOLs with simple decentration are usually satisfactorily managed by observation. Observation also may be recommended even for luxation if other superseding medical or ocular problems prohibit further surgery, or if the patient simply elects not to pursue further surgery. Occasionally, management with topical miotics can be visually beneficial, especially for minor subluxations. In a series of 15 patients with dislocated anterior chamber or iris plane IOLs that were observed, a visual acuity of 20/40 was reported in 60%, but retinal detachment occurred in two patients.39
Removal or Exchange
The IOL is usually exchanged when there is damage to the IOL during surgical management (e.g., broken haptic), if available instrumentation to effect repositioning is lacking, or if highly flexible haptics, or polypropylene haptics that are appreciably misshapen, make the IOL unsuitable for sulcus fixation.
One encountered circumstance in which IOL exchange may be considered occurs in certain cases with silicone IOLs.46 Silicone IOLs are slippery and more difficult to grasp than polymethylmethacrylate (PMMA) IOLs, but can usually be engaged and elevated from the retinal surface with a vitreoretinal pick or a lighted pick. A serrated or diamond-dusted forcep may be necessary. Caution should be exercised to avoid scratching the center of the optic during attempted repostioning. Silicone plate haptic IOLs are extremely floppy and difficult to manipulate. They are designed specifically for capsular bag fixation. Damage to the silicone optic or presence of a plate haptic lens that cannot be repositioned within the capsular bag will require IOL exchange.45,47
In patients for whom repositioning PC IOLs proves problematic, an intraoperative decision can be made to remove and exchange it with an AC IOL or scleral suture fixated PC IOL. Exchange for a suture-fixated PC IOL has been simplified by the availability of IOL designs that include holes (eye-lets) in the haptics. However, explanting and reimplanting an IOL may risk more corneal endothelial cell trauma as compared to repositioning techniques. Exchange for an AC IOL may be less traumatic to the corneal endothelium and may be easier and faster to accomplish. Newer AC IOL designs reportedly avoid complications caused by the mechanical side effects of earlier AC IOL designs compared to PC IOLs, and the results can presumably be extrapolated to dislocated IOL management.48 In general, scleral suture fixation with a PC IOL is preferred by the authors over AC IOL implantation.45 In any case, it is important that the possibility of either PC IOL or AC IOL implantation should be anticipated with proper IOL power calculations, and IOL availability, before surgery.
An ancillary option is observation of the dislocated IOL, in which case visual rehabilitation is achieved with implantation of a second (usually AC) IOL.49–51 This should be considered an option of last resort, however, as most patients are concerned with the presence of a dislocated lens implant.
Intraocular Lens Repositioning
IOL repositioning completes the initial surgical objectives of the cataract surgery and is the most commonly elected surgical approach. There are three basic approaches to IOL repositioning: (1) IOL repositioning without sutures using residual peripheral anterior or posterior capsule, (2) iris sutured fixation, and (3) scleral suture fixation.
Subluxated IOLs associated with an intact, or mostly intact, posterior capsule may be repositioned from an anterior approach if there is only moderate subluxation. Usually at least one haptic is posteriorly malpositioned—either protruding through an unseen zonular dehiscence in an area without posterior capsular support or posterior to the residual capsule. A pars plana approach is optimal for patients with large posterior capsule defects, for patients with IOL luxation into the vitreous cavity, and for patients with coexisting ocular complications such as retinal detachment.
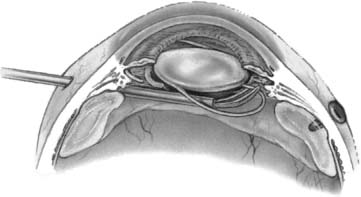
Recognition and use of adequate capsular support are as important for repositioning the PC IOL as they are for primary placement. Generally, the IOL remains well supported if at least 180 degrees of peripheral capsular material is intact. More extensive support is necessary, however, when the inferior capsule is absent or if the margin of the residual capsule where IOL haptics are to be placed is of questionable integrity. Repositioning by capsular fixation is the most common management technique in reported series38–45 and is the authors’ first choice when technically possible. Surgical success depends on accurate placement of the haptics into the ciliary sulcus, which requires visualization of the residual capsule.52,53 Placement of iris hooks is useful in selected cases, but usually strategic local iris retraction with a hooked instrument allows confident visualization. A useful maneuver in a pars plana approach is to bring the IOL anteriorly and capture at least one haptic anterior to the iris (Fig. 26–3). After the IOL is stabilized in the anterior chamber, the second haptic can be guided between the residual capsule and posterior iris surface either by rotating the lens or by grasping the haptic with an intraocular forceps via the pars plana. Because of the widespread use of capsulorrhexis, the peripheral anterior capsule is usually intact and serves as an effective interface for sulcus fixation (Fig. 26–3). Repositioning a PC IOL permanently into the anterior chamber also has been reported, but is not recommended because of chronic chafing of the iris by the IOL and lens power considerations.54
FIGURE 26–3 When repositioning a posterior chamber (PC) intraocular lens (IOL) onto residual capsular remnants, it may be useful to bring one haptic anterior to the iris to facilitate accurate visualization of haptic placement over residual capsule. (From Regillo CD, Brown GC, Flynn HW Jr. Vitreoretinal Disease: The Essentials. New York: Thieme; 1999:572, Fig. 35–7.)
Iris fixation sutures were initially described for the use of dislocated AC IOLs.55 However, their use has been modified for fixation of dislocated posterior chamber implants using a limbal or a pars plana approach.52,56 This technique requires that a suture pass through the cornea, iris, around the IOL haptic, and back out through the iris and cornea. Because accurate placement of the needle is difficult, it is challenging to optimize IOL centration. Also, concern regarding iris-mediated chronic inflammation and the technical difficulty encountered during suture placement have led to the development of other techniques.
Scleral fixation sutures were first introduced for implantation of secondary IOLs and for primary IOL placement in the absence of satisfactory peripheral capsular support in a limbal or pars plana approach.21–32 Early reports described pulling the haptic to or externally through57–59 a sclerotomy to position a suture on the haptic before suturing to the deep part of the sclerotomy wound. Subsequently, IOL repositioning using transscleral fixation sutures via a pars plana approach mimicking the techniques of secondary IOL fixation was described. Numerous innovative modifications have made the technique easier and safer and will be reviewed below. Components common to all scleral suture fixation techniques include (1) retrieving the IOL, (2) introducing a suture loop through the ciliary sulcus region into the vitreous cavity, (3) passing the suture loop around the IOL haptic, (4) securing the suture to the sclera, and (5) covering or burying the scleral suture knot. A wide variety of techniques have been described to achieve these goals.
Most proposed techniques modify how the suture loop is introduced and attached to the IOL haptic. Such techniques have included imbricating the IOL haptic into the sutures used to close the sclerotomy,60 externalizing the haptics to attach a suture,61 using a needle guide to thread the suture around the haptic,62 introducing a small needle intraocularly to capture the haptic,63 suturing through IOL optic positioning holes,64,65 backing a large needle into the eye to introduce a suture loop,66 grasping a loop by intraocular forceps,67,68 and introducing the suture from a third sclerotomy.69 Other proposed variation techniques include achieving three- or four-point fixation to lessen lens torsion,70 using specially designed small-gauge forceps to aid in maneuvering the loop around the haptic,71 and using perfluorocarbon liquids to place the implant in a convenient position for suturing.40,72,73 Most posterior segment surgeons find the use of perfluorocarbon liquids unnecessary.
The current technique preferred by the authors will be described and illustrated.74 A standard three-port pars plana vitrectomy is performed to remove the formed vitreous and to mobilize the IOL. Partial-thickness, limbal-based scleral flaps are dissected, most conveniently in the 1 and 7 o’clock meridians to avoid the previous cataract wound and yet be accessible. A disposable 27-gauge needle75 (Escalon-Trek Medical, Milwaukee, WI) with a hole located in the bevel is threaded with 9–0 polypropylene suture and introduced into the eye 1 mm posterior to the limbus in the bed of the partial-thickness scleral flap (Fig. 26–4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree