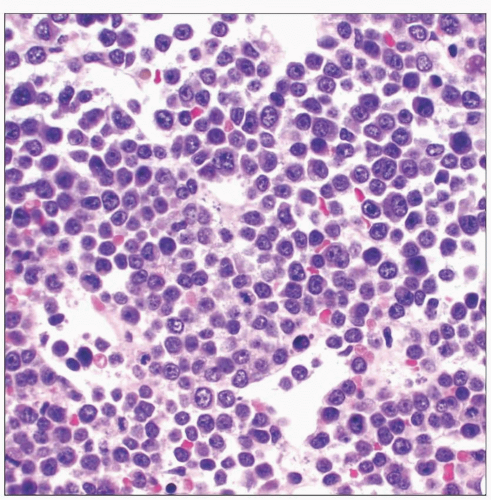
Post-Transplant Lymphoproliferative Disorders in Bone Marrow
Mohammad A. Vasef, MD
Key Facts
Etiology/Pathogenesis
Risk factors of PTLD in solid organ recipients
Epstein-Barr virus (EBV) serology status
Type of organ transplanted
Lowest risk in renal allograft recipients
Highest risk in intestinal recipients
Type and intensity of immunosuppressive regimen
Higher risk with anti-CD3 or anti-thymocyte globulin (ATG) administration
Increased risk with high-intensity immunosuppression
Age of patient
Higher risk in pediatric allograft recipients
Risk factors of PTLD in allogeneic HSCT
Overall lower risk in HSCT compared to SOT
Increased risk of early-onset PTLD with unrelated or HLA-mismatched related donors
Increased risk of PTLD with T-cell depleted donor marrow transplantation
Microscopic Pathology
Early lesions
Polymorphic PTLD
Monomorphic PTLD, B-cell neoplasms
Monomorphic PTLD, T-cell neoplasms
Classical Hodgkin lymphoma
Top Differential Diagnoses
Infectious mononucleosis
Iatrogenic lymphoproliferative disorders
De novo lymphoid neoplasms
Allograft rejection or infection
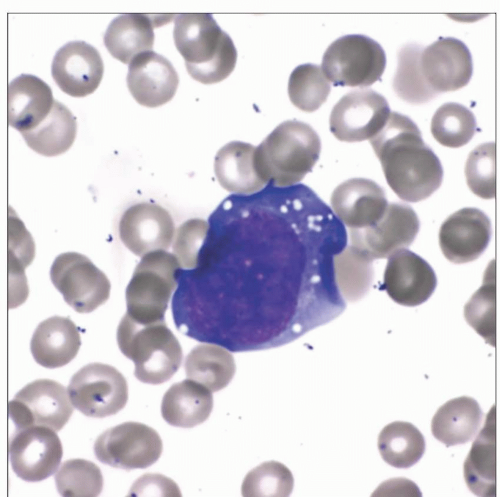 Wright-stained peripheral blood smear of an adult male with a history of liver transplantation demonstrates a rare large circulating atypical lymphoid cell with fine cytoplasmic vacuoles. |
TERMINOLOGY
Abbreviations
Post-transplant lymphoproliferative disorders (PTLD)
Definitions
Lymphoid proliferation secondary to immunosuppression in setting of allogeneic hematopoietic stem cell transplantation (HSCT) or solid organ transplantation (SOT)
ETIOLOGY/PATHOGENESIS
Risk Factors of PTLD in SOT
Epstein-Barr virus (EBV) serology status
20-50x higher risk for PTLD development in seronegative recipient from seropositive donor
Significantly higher risk in children due to frequent EBV-naive pretransplant status
Primary EBV infection in transplant children is commonly acquired from donor organ
Lower risk in adults due to EBV reactivation rather than primary EBV infection
Type of organ transplanted
Lowest risk in renal allograft recipients
Highest risk in intestinal recipients
High rate of PTLD in intestinal transplants may be due to large quantity of lymphocytes in intestine allograft
Type and intensity of immunosuppressive regimen
Higher risk in individuals receiving anti-CD3 or anti-thymocyte globulin (ATG)
Significant increased risk in individuals receiving high-intensity immunosuppression
Age of patient
Significantly higher risk of PTLD in pediatric organ recipients compared to adults
Higher risk of PTLD in children is attributable to primary EBV infection after transplantation
Risk Factors of PTLD in Allogeneic HSCT
Overall much lower risk in hematopoietic stem cell recipients compared to solid organ recipients
Majority of PTLD cases occur < 1 year after transplantation
Increased risk of early-onset PTLD with unrelated or HLA mismatched related donors
Increased risk of PTLD with ATG therapy for graft-vs.-host disease prophylaxis
Increased risk of PTLD with T-cell depleted donor marrow transplantation
CLINICAL ISSUES
Epidemiology
Incidence
Significant variability in incidence ranging from < 1% to > 20%
< 1% in renal transplant patients
2-3% in heart or liver transplant patients
5-10% in lung or heart-lung recipients
20% in intestinal transplant recipients
1% in hematopoietic stem cell recipients
Site
Involvement of nodal and extranodal sites common in PTLD in all types of allograft transplantation
Gastrointestinal tract, lungs, and liver are common extranodal sites of involvement by PTLD
Central nervous system involvement by PTLD is rare
PTLD frequently involves allograft in solid organ transplantation except in heart allograft
Tonsils and adenoids are commonly involved sites in early PTLD lesions
Overt bone marrow involvement is uncommon
Peripheral blood involvement by PTLD is rare
Disseminated disease more common in allogeneic bone marrow transplantation
Presentation
Clinical presentation of PTLD highly variable
Patients with PTLD may be asymptomatic
Some patients present with fever and weight loss
PTLD in children may present with features mimicking infectious mononucleosis
Tonsillitis &/or lymphadenopathy may be present
PTLD can develop at any time post transplantation
Early-onset PTLD
Develops within 1 year following transplant
Late-onset PTLD
Develops > 1 year after transplant
Accounts for approximately 40% of all PTLD cases
PTLD occurs after 5 years post transplantation in < 10% of transplant patients
Treatment
Reduction in immunosuppressive medications
Initial step in management of PTLD
Needs to be balanced against risk of graft rejection
Rapid reduction in immunosuppression more practical in liver and renal transplant patients
Factors predictive of poor response to immunosuppressive reduction
Elevated lactate dehydrogenase (LDH)
Organ dysfunction at diagnosis
Multi-organ involvement by PTLD
Immunosuppressive reduction may not be effective in monomorphic and some polymorphic PTLD
Anti-CD20 monoclonal antibody (rituximab) therapy
Historically used as single agent after failure of immunosuppressive reduction
35-70% complete remission rates reported in PTLD cases after solid organ transplantation
Improved response rate also reported in PTLD after hematopoietic stem cell transplantation
Predictive factors of response to rituximab
EBV-positive PTLD
Shorter interval from transplantation to development of PTLD
Normal LDH level
Limited numbers of involved sites

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree