Positioning the Patient for Surgery
Pauline Anne Heizenroth
Proper patient positioning is essential for safe, successful surgery procedures. The surgical team ensures uncompromised and physiologically safe patient positioning by understanding the systems affected by positioning and their associated risks. Given that surgery may be performed on all anatomic areas, body positioning may result in multiple and sometimes unnatural configurations in order to expose a surgical site. Positioning, combined with anesthesia and its physiologic effects, can yield harmful changes if safety factors are not considered as a paramount patient safety outcome. This chapter discusses some of the risks and prevention strategies associated with standard surgical positions.
The goals of surgical positioning include providing optimal exposure and access to the surgical site, maintaining body alignment, supporting circulatory and respiratory function, protecting neuromuscular and skin integrity, and allowing access to intravenous (IV) sites and anesthesia support devices. Meeting these goals while simultaneously maintaining patient comfort and safety is the responsibility of every member of the surgical team.
Anatomic and Physiologic Considerations
The perioperative nurse must understand thoroughly the anatomic and physiologic changes associated with positioning the patient. These changes are affected by numerous factors, such as the type of surgical position; the length of time the patient is in that position; the operating room (OR) bed, padding, and positioning devices used; the type of anesthesia given; and the operative procedure. These changes most frequently affect: (1) the skin and underlying tissue; (2) the musculoskeletal system; (3) the nervous system; (4) the cardiovascular system; (5) the respiratory system; and (6) other vulnerable areas, such as the eyes, breasts, perineum, and fingers.
Skin and Underlying Tissue
Physical forces used to establish and maintain a surgical position can injure the skin and underlying tissue. These forces include pressure, shear, and friction. Additionally, OR conditions such as moisture, heat, cold, and negativity further increase the vulnerability of the skin and underlying tissues to injury.
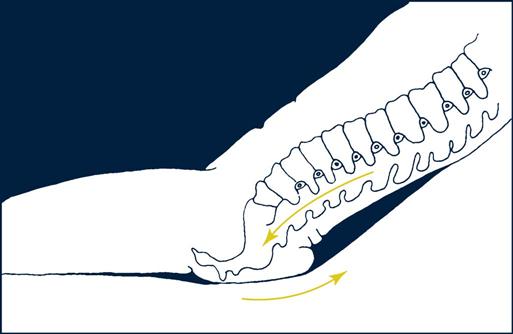
• Shear is the folding of underlying tissue when the skeletal structure moves while the skin remains stationary. A parallel force creates shear compared with a perpendicular force created by pressure (Figure 6-1). This happens when the head of the bed is raised or lowered and when the patient is placed in Trendelenburg (head down, supine) or reverse Trendelenburg (head up, supine) position. As gravity pulls the skeleton down, the stretching, folding, and tearing of the underlying tissues as they slide with the skeleton occlude vascular perfusion, which leads to tissue ischemia.
Pressure Ulcers.
The National Pressure Ulcer Advisory Panel (NPUAP) defines a pressure ulcer as “a localized injury to the skin and/or underlying tissue, usually over a bony prominence, as a result of pressure or pressure in combination with shear and/or friction” (NPUAP, Pressure Ulcer Stages/Categories, 2013). In 2007 the NPUAP revised its Guidelines for Staging Pressure Ulcers to include two additional stages: “deep tissue injury” and “unstageable.” Table 6-1 reviews current NPUAP Guidelines for Staging Pressure Ulcers.
TABLE 6-1
NPUAP Guidelines for Staging Pressure Ulcers
| Stage/Category | Definition |
| Deep tissue injury | Purple or maroon localized area of discolored intact skin or blood-filled blister due to damage of underlying soft tissue from pressure and/or shear. The area may be preceded by tissue that is painful, firm, mushy, boggy, warmer or cooler as compared to adjacent tissue. Deep tissue injury may be difficult to detect in individuals with dark skin tones. Evolution may include a thin blister over a dark wound bed. The wound may further evolve and become covered by thin eschar. Evolution may be rapid, exposing additional layers of tissue even with optimal treatment. |
| Stage I | Intact skin with nonblanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its color may differ from the surrounding area. The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue. Stage I may be difficult to detect in individuals with dark skin tones. May indicate “at risk” persons. |
| Stage II | Partial-thickness loss of dermis presenting as a shallow open ulcer with a red-pink wound bed, without slough. May also present as an intact or open/ruptured serum-filled or serosanguineous-filled blister. Presents as a shiny or dry shallow ulcer without slough or bruising.* This category should not be used to describe skin tears; tape burns; incontinence associated dermatitis, maceration, or excoriation. |
| Stage III | Full-thickness tissue loss. Subcutaneous fat may be visible, but bone, tendon, or muscle is not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunneling. The depth of a stage III pressure ulcer varies by anatomic location. The bridge of the nose, ear, occiput, and malleolus do not have (adipose) subcutaneous tissue, and stage III ulcers can be shallow. In contrast, areas of significant adiposity can develop extremely deep category/stage III pressure ulcers. Bone/tendon is not visible or directly palpable. |
| Stage IV | Full-thickness tissue loss with exposed bone, tendon, or muscle. Slough or eschar may be present. Often includes undermining and tunneling. The depth of a category/stage IV pressure ulcer varies by anatomic location. The bridge of the nose, ear, occiput, and malleolus do not have (adipose) subcutaneous tissue, and these ulcers can be shallow. Stage IV ulcers can extend into muscle and/or supporting structures (e.g., fascia, tendon, or joint capsule) making osteomyelitis or osteitis likely to occur. Exposed bone/muscle is visible or directly palpable. |
| Unstageable | Full-thickness tissue loss in which actual depth of the ulcer is completely obscured by slough (yellow, tan, gray, green, or brown) and/or eschar (tan, brown, or black) in the wound bed. Until enough slough and/or eschar is removed to expose the base of the wound, the true depth cannot be determined, but it will be either a stage III or IV. Stable (dry, adherent, intact without erythema or fluctuance) eschar on the heels serves as the body’s natural (biologic) “cover” and should not be removed. |
*Bruising indicates deep tissue injury.
Modified from National Pressure Ulcer Advisory Panel (NPUAP): NPUAP Pressure ulcer stages/categories, 2013, available at www.npuap.org/resources/educational-and-clinical-resources/npuap-pressure-ulcer-stagescategories/. Accessed April 27, 2013. Used with permission of the National Pressure Ulcer Advisory Panel.
In 2008 the Centers for Medicare & Medicaid Services (CMS) decided to end reimbursement to hospitals for many preventable hospital-acquired conditions, which it called “never events.” Among these conditions were stage III or IV pressure ulcers that develop after admission (CMS, 2008). This highlights the need to assess the skin thoroughly before surgery. Proper skin assessment includes temperature, color, moisture level, turgor, and integrity. Inarguably, the costs of treating pressure ulcers are far more than the costs of preventing them. Hospitals must therefore focus on preventive strategies that take into account the extrinsic and intrinsic factors that interact to contribute to the overall risk of developing pressure sores.
Extrinsic Factors.
Pressure is the major physical force responsible for pressure ulcer formation. Its intensity and duration affect the ultimate outcome of whether the tissue suffers damage. An inverse relationship exists between pressure and time: the greater the pressure, the shorter time it takes to cause ischemic changes. Pressures greater than 32 mm Hg (capillary interface pressure) can occlude flow of the arterioles, which nourish and oxygenate the tissue at the capillary level. An individual can endure relatively large amounts of pressure for a short time or smaller amounts of pressure for a longer time without sustaining tissue damage. A time limit can be reached, however, when the tissue no longer can withstand the pressure (especially >32 mm Hg) and cells die from lack of oxygen and nutrients. Such prolonged pressure can result in tissue ischemia manifested as a pressure ulcer.
The intensity of pressure depends on the size of the surface area being compressed. The smaller the surface area in which pressure is applied, the greater the force (pressure per surface area). Compression of tissue between an external surface and a bony prominence of the body tends to create high forces of pressure in the local tissue surrounding that bone. Pressure ulcers most often develop around bony prominences such as the heels, elbows, and sacrum in the supine position. Often the tissue injury is most severe in deep areas immediately adjacent to bone. As deeper ischemia progresses outward toward the skin, the damage may not be visible for several days. Theoretically, if pressure could be distributed more evenly over other surfaces of the body, the force of pressure in those vulnerable areas would lessen. This theory fuels manufacturers to create OR mattress overlays and padding materials designed to distribute forces of pressure more evenly.
The greater the amount of time a patient is on the OR bed, the higher the risk of OR-acquired pressure ulcer development when combined with the multiple variables of the intraoperative experience. Duration is considered more of a causative factor than is the intensity of pressure. Healthy individuals may tolerate high external pressures (e.g., 100 mm Hg) over bony prominences for short periods, but longer, constant, unrelieved pressure tends to cause microscopic necrosis. Lowered pressures over extended periods also may cause tissue damage, particularly if tissue tolerance is diminished. Frequent repositioning of an immobilized patient can protect against or lessen adverse effects of pressure. Repositioning a patient every 2 hours has long been the standard for lessening the negative effects of pressure on the immobile patient. However, OR patients cannot be repositioned without jeopardizing access to the operative site and the integrity of the sterile field. Case-based exceptions might include limited repositioning of extremities and/or the head when such movement would not interfere with the surgical procedure or the sterile field. The perioperative nurse should plan ahead to ensure that preventive measures are taken to reduce pressure ulcer risks. Pressure-relieving OR mattresses or overlays may reduce pressure in dependent areas. Padding applied around rigid surfaces in contact with the patient and suspending vulnerable areas off the OR bed can reduce localized pressure.
Patients undergoing regional or general anesthesia are at increased risk because normal muscle tone and pain sensations that enable the body to adjust itself in response to excessive pressure are disabled. Patients often are placed in positions that they would not be able to tolerate comfortably if they were awake or even asleep without the use of anesthetics. Anesthesia also causes hypotension, which can reduce tissue perfusion and thus reduce the exchange of oxygen and carbon dioxide. Connor and colleagues (2010) found that prolonged hypotension is a valid predictor of pressure ulcer risk in the OR. All these anesthetic effects can heighten the effect of pressure forces, particularly if other comorbid conditions are present.
A study by Fred and colleagues (2012) shows that patients with at least a 1° F (1.8° C) drop in temperature may be at increased risk for intraoperatively acquired pressure ulcers. Hypothermia is the frequent result of the cooler OR environment, exposure of external and internal body surfaces, and infusion and irrigation with unwarmed solutions. This causes peripheral vessels to constrict to conserve core temperature and increases the patient’s heat metabolism. This increased metabolism leads to increased tissue needs for oxygen, nutrients, and metabolic waste-product removal. When tissue is under pressure, these needs are not easily met. When hypothermic patients later emerge from general anesthesia, they often shiver, which further increases their oxygen requirements and may increase their risk for pressure ulcers as well as myocardial infarction. Minimizing intraoperative hypothermia thus is imperative.
Ways to offset hypothermia in the OR and to reduce its detrimental effects include using forced-air warming therapy over the patient. Avoid placing a warming blanket under the patient in the area where pressures are greatest because tissue requirements tend to increase there and the effect of tissue ischemia may be more profound. Minimize unnecessary skin exposure and use warm irrigation fluids, especially deep inside the body cavity. To warm IV fluids, insert a fluid-warming coil or place most of the IV fluid tubing under the forced-air warming blanket.
The type of surgery performed can affect pressure ulcer development. Patients who undergo cardiac, general, thoracic, and vascular procedures tend to be at greater risk, possibly because these cases immobilize patients for long periods (Denholm, 2008). In addition, procedures that involve significant blood loss, extracorporeal circulation, and clamping of major vessels can contribute to the lack of blood flow to tissue under pressure.
The type of positioning devices used can increase pressure ulcer development risk as well. Rigid, unpadded objects that press against a body surface, such as stirrup bars, compressed beanbags, or rolled sheets, can cause tissue injury if pressure is not relieved periodically.
Negativity, as explained earlier, can override the pressure-relieving capabilities of mattresses and padding. Placing a warm blanket under a patient may be soothing initially, but if a surgical procedure is long, pressure to the bony prominences resting on the blanket will be greater than if only a sheet and drawsheet are used. Additionally, wrinkles and folds can cause further pressure points.
When a patient is moved into a shear-producing position (e.g., from supine to semi-Fowler) before being prepped and draped, the OR team can take measures to reduce shear forces. One technique is to slightly move the patient’s back away from the mattress momentarily to allow the skin to realign with its surrounding skeletal structures. When the patient is placed in these positions during a surgical procedure, however, interventions to reduce shear forces are limited. Reducing the time that shear forces are in place is the best measure to reduce tissue injury.
Intrinsic Factors.
Intrinsic factors lower a patient’s tissue tolerance to pressure and decrease the time and pressure required for tissue breakdown. Certain preexisting conditions are regarded as intrinsic risk factors for OR-induced pressure ulcer development. These conditions include diabetes mellitus, smoking, peripheral vascular disease, cerebral vascular disease, urinary or fecal incontinence, anemia, malignancy, sepsis, steroid use, morbid obesity, malnutrition (serum albumin levels less than 3.5 g/dL), advanced age, body size (obesity as well as thin, frail build), and impaired mobility (Connor et al, 2010; Cox, 2011; Denholm, 2008; Lyder and Ayello, 2008; Munro, 2010; Primiano, 2011; Walton-Geer, 2009).
Various scales exist to determine pressure ulcer potential. One widely used is the Braden Scale, which scores a patient’s potential for pressure sore development based on various intrinsic factors. It is valid and reliable in acute care and long-term care settings and has been used for many years to guide nursing interventions. It does not take into account, however, various extrinsic factors patients are subjected to in critical care areas such as the OR or the intensive care unit (ICU), in both of which immobile patients are completely dependent on caregivers to sustain their fragile hemodynamic status and where routine, timely repositioning may be contraindicated.
Recent studies have tested the relevance of the Braden Scale in the perioperative setting (Connor et al, 2010; Galvin and Curley, 2012) and the medical-surgical ICU setting (Cox, 2011, Suriadi et al, 2008). These studies concluded that not all Braden Scale risk factors are predictable in these patients and that other significant risk factors are not identified on Braden or other pressure ulcer predictive scales used in hospital settings. The Braden score may be used as a preoperative baseline, but the OR is unique when compared with other areas of the hospital. Typical predictors of pressure ulcer risks may not work once anesthetized, immobile patients are put in atypical positions for varying lengths of time, which may disrupt their cardiovascular, hemodynamic, and thermoregulation statuses. This potential disruption underscores the suggestion by Walton-Geer (2009) and others that all surgical patients should be considered at risk to develop pressure ulcers. Some patients are at a higher risk than others, and risk assessment scales need to be developed specific to the perioperative patient. Several nursing studies (Connor et al, 2010; Galvin and Curley, 2012; Primiano et al, 2011) have yielded perioperative variables usable to predict pressure ulcer development (Research Highlight). Some OR teams have developed their own perioperative risk assessment tools (Mathias, 2008; Munro, 2010), but these have not as yet been widely tested for validity and reliability.
Over the past decades multiple other studies with surgical patients have attempted to determine risk factors specific to this patient population. The problem with attempting to draw out distinct perioperative risk factors for pressure ulcer development that would apply on a general scale is that so many exist; risk factors vary substantially from hospital to hospital as to type and length of procedures, types of anesthesia, positions, positioning devices, mattress surfaces, patient warming methods, and unplanned surgical and anesthetic events, along with multiple and varying intrinsic factors such as age, weight and comorbidities. Even so, given our extant knowledge coupled with future research, a baseline perioperative pressure ulcer risk scale that could be individualized for each patient and situation would serve as a useful guide to perioperative nurses to plan and care for this patient population. Development of such a prospective scale, particularized for each surgical patient, remains an incomplete goal and unmet need of perioperative nursing.
Assessment for pressure ulcer risk factors and development nevertheless should be done consistently and aimed at three periods: preoperative, intraoperative, and postoperative. Such assessments should accompany the hand-off report to the nurse in the postanesthesia care unit (PACU) receiving the patient (see Chapter 10). Included in postoperative assessment is inspection for changes in skin temperature, color, moisture level, turgor, and integrity. These inspections start in the preoperative area and OR, continue in the PACU, and go beyond to discharge. It is in the early postoperative phase that many small problems picked up by astute assessments can be treated to avert bigger problems.
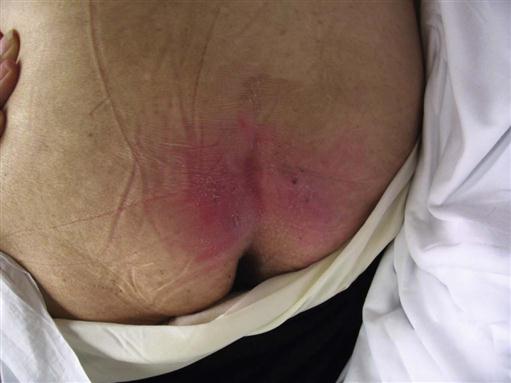
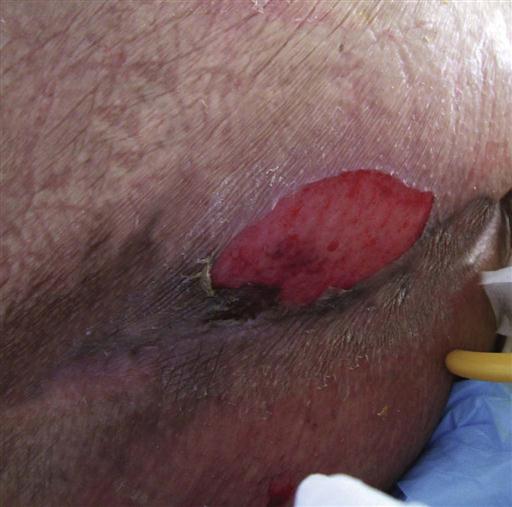
Stage I or stage II pressure ulcers (Figures 6-2 and 6-3) may be evident immediately after surgery. These areas must be kept free from additional pressure for healing to occur and to keep them from advancing to higher stages. Such ulcers are sometimes misidentified and treated inappropriately. Stage I pressure ulcers can look like normal erythema, a reaction to pressure in which the healthy tissue blanches when compressed and generally fades on its own in half to three quarters of the time that the area was under pressure, with little or no permanent tissue damage. Stage I pressure ulcers do not blanch when compressed. Failure to blanch is a serious sign, indicating that the compromised tissue is not receiving adequate oxygen and nutrients. The blistering or partial dermal loss that occurs in stage II pressure ulcers is often misidentified as a chemical or thermal burn. The lack of contact with a chemical or thermal agent in that area and its location under a bony prominence or near where a hard surface had been during surgery can help distinguish it as a serious stage II pressure ulcer.
Full manifestation of pressure ulcers may not occur from hours to days after the triggering injury. Given such delayed manifestation, the connection to the triggering event (the surgical experience) may be overlooked. Lack of awareness of an early pressure injury can lead to a delay of pressure relief and treatment. Inappropriate treatment can contribute to avoidable advancing stages of pressure ulcers.
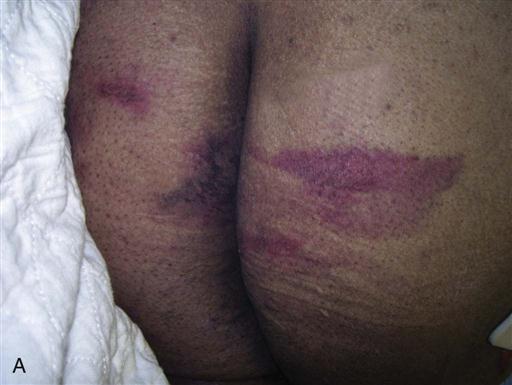
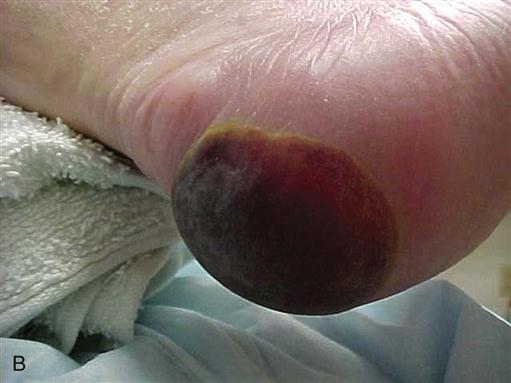
Deep tissue injury (DTI) (Figure 6-4) must also be differentiated from a stage I or II pressure ulcer. Stage I ulcers present with discolored, nonblanchable, but intact skin, which is also true of a DTI. Instead of pink or red, however, the DTI is purple or maroon in appearance, like that of a deep bruise. Stage II ulcers present as an intact or open/ruptured serum filled blister, whereas a DTI blister fills with blood. The NPUAP added the DTI category to help identify a dangerous form of pressure ulcer in which deeper tissues are subjected to such a long duration of ischemia that reperfusion causes additional microvascular injury (reperfusion injury). What began in appearance as a closed discolored area rapidly deteriorates to a full-thickness stage III or IV pressure ulcer, even with optimal treatment. The epidemiology, clinical course, and efficacious treatment of DTIs require more research (NPUAP, Deep Tissue Injury, 2013).
Pressure alopecia (pressure-induced hair loss on the scalp) is a complication not typically classified as a pressure ulcer, but can result from prolonged local pressure to the scalp during and after surgery. Symptoms can occur between postoperative days 3 and 28. Occipital scalp pain, swelling, exudate, crusting, or focal ulceration may precede the actual loss of hair. Pressure alopecia most commonly occurs in procedures that require prolonged intubation and head immobilization. In cardiac surgery, for example, the anesthesia induction-to-extubation time may extend more than 24 hours. The longer the time, the greater the risk that alopecia may be permanent. Repositioning the patient’s head every 30 minutes using soft, contoured head padding can reduce the potential for pressure alopecia.
Musculoskeletal System
The musculoskeletal system may be subjected to unusual stress during operative positioning. Ordinarily the alert patient maintains normal range of motion by pain and pressure receptors that warn against overstretching and twisting of ligaments, tendons, and muscles. The tone of opposing muscle groups also acts to prevent strain and stretch to muscle fibers. In surgery, however, when pharmacologic agents such as anesthetics and muscle relaxants depress pain and pressure receptors, and muscle tone, a patient’s normal defense mechanisms cannot guard against joint damage and muscle stretch and strain. A patient safety goal is to maintain the body’s natural alignment as much as possible, while still providing adequate access to the surgical site. The perioperative team needs to be aware of the limits to range of motion, refraining from joint extension beyond what is necessary.
Every time a patient is transferred to or from the OR bed, alignment of the patient’s body needs to be maintained. This is especially true when transfer involves moving the patient from a supine position to lateral or prone. During positional changes, extremities must be gently supported; quick, jerky movement of unsupported limbs can cause strain to the muscles and ligaments.
Nervous System
Nervous system depression accompanies the administration of anesthetic agents and other drugs. The degree of depression depends on the type of regional anesthesia or the level of general anesthesia. Pain and pressure receptors may be affected regionally or systemically. The most important factor for perioperative nurses to remember is that when nervous system depression occurs, the body’s communication and command system becomes totally or partially ineffective. Compensatory reactions to changes in physical status no longer respond normally. Lifesaving, physiologic adaptive mechanisms are altered. The body can no longer compensate for the stresses of surgical positioning.
Peripheral Neuropathies.
Peripheral nerves can suffer injury during positioning, resulting in impaired sensory function or motor function, or both. The mechanisms for nerve injuries include compression (pressure), stretch, direct trauma, laceration, ischemia, and metabolic derangement (Prielipp and Warner, 2009; Saidha et al, 2010). Stretching and compression tend to be the main culprits in position-induced nerve injuries. Prolonged stretching from hyperabduction of an extremity or compression from pressure results in ischemia, which can progress to necrosis. In addition, collateral damage to the surrounding tissue and capillaries can affect circulation and nourishment to nerves. These pathologic forces combine to result in structural or functional damage to the nerves.
There are three degrees of nerve injury. Depending on degree, subsequent injuries may be temporary or permanent. The first degree of injury, neuropraxia, is a response to compression and has the best prognosis. There is only slight demyelination and no axonal degeneration. Full recovery can be expected within 6 weeks without residual loss of function. The second degree of injury, axonotmesis, manifests as the destruction of the axons within a nerve sheath that remains intact. The distal axons degenerate, but proximal axons are able to use the intact sheath to guide regeneration at a rate of 1 mm per day. The third and most severe degree of injury is neurotmesis in which disruption to the axon, sheath, and connective tissue covering are disrupted. Again, the distal axon degenerates. Without an intact sheath to guide regeneration, regrowth is disorganized, sometimes forming painful neuromas. Surgical resection of the axon ends with reapproximation is the only way to help regain function (Ellsworth et al, 2009).
During surgery all patients are at risk for nerve injury if sustained compression is placed on nerves. Numerous patient characteristics have been identified, however, that potentially increase the risk for intraoperatively acquired neuropathies. These include diabetes mellitus, peripheral vascular disease, electrolyte imbalances, vitamin B12 deficiency, alcoholism, smoking, malnutrition, advanced age, previous nerve injuries or neuropathies (e.g., carpal tunnel syndrome, thoracic outlet syndrome), arthritis, obesity, thin body habitus, and male gender (for ulnar nerve injuries) (Edgecombe et al, 2008; Renter and Beissel, 2013; Shveiky et al, 2010; Uribe et al, 2010; Welch et al, 2009). Possible risk factors associated with the patient’s surgical experience include lengthy surgical procedures, the use of abdominal pelvic self-retaining retractors, long duration in the lithotomy position, sternal retraction from a median sternotomy, surgical procedures in which shoulder braces are used for support while the patient is in steep Trendelenburg, improper arm placement, tight arm restraints, improperly placed blood pressure cuff, prolonged hospitalization, hypotension, hypothermia, and prolonged bed rest postoperatively (Akhavan et al, 2010; ASA, 2011; Cassorla and Lee, 2010; Shveiky et al, 2010; Warner, 2009). Damage usually remains undiscovered until the patient reaches the PACU or even days or weeks after the suspected insult. This delay can lead to some confusion as to whether the injury occurred during surgery or convalescence. Although the incidence is relatively low, claims of peripheral nerve injuries are a significant source of professional liability claims.
Upper Extremity Neuropathies.
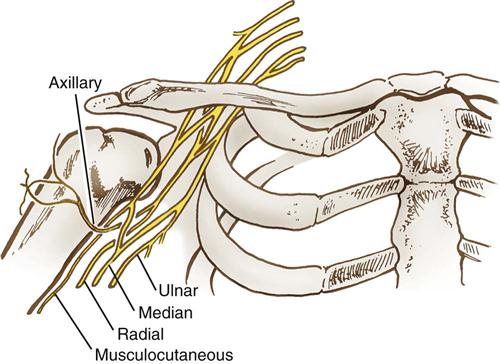
Upper extremity neuropathies generate from lesions to the brachial plexus and the nerves that emerge from it. The brachial plexus consists of a bundle of nerve cords that run through the shoulder and innervate the lower shoulder, arm, and hand (Figure 6-5). This bundle originates from cervical spinal nerves C5-C8. The nerve cords emerge to form peripheral nerves. The axillary nerve innervates the deltoid muscle. The musculoskeletal nerve innervates the biceps muscle. Three main peripheral nerves run down the arm to the fingers: median, radial, and ulnar (Figure 6-6).
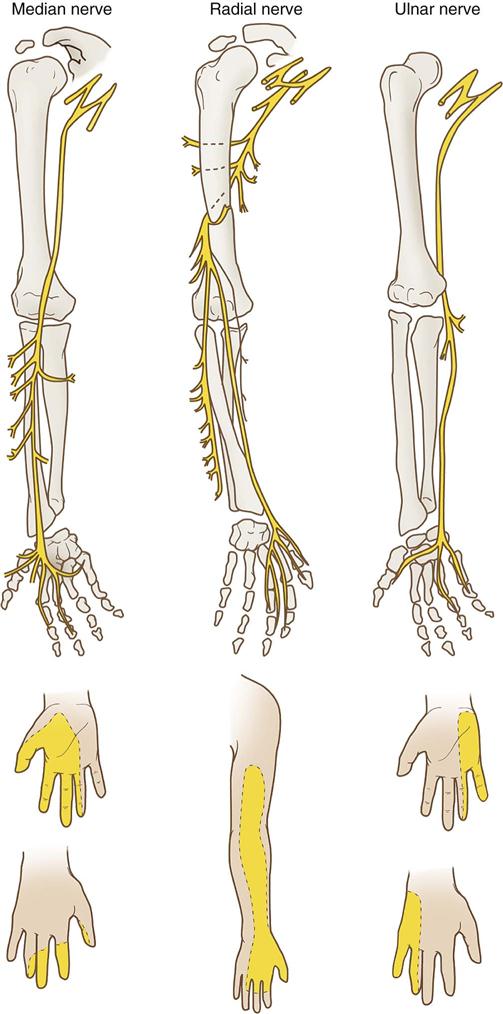
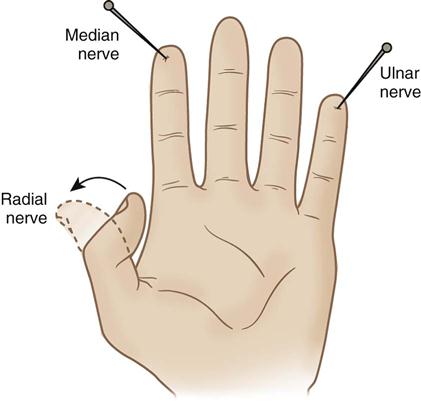
The median nerve runs through the antecubital space to the fingers and provides sensory and motor ability to the distal and palmar surfaces of the thumb and adjacent two fingers. A normally perceived pinprick to the palmar surface of the distal index finger generally would show that the median nerve was intact (Figure 6-7).
The radial nerve loops around the posterior humerus before it runs through the antecubital space to the fingers. It provides motor and sensory function to the triceps and the muscles of the posterior forearm and hand. Injury to this area can cause difficulty extending the wrist and thumb. If the distal thumb can be actively extended, this generally indicates an intact radial nerve (see Figure 6-7).
The ulnar nerve runs behind the elbow and innervates the third, fourth, and fifth fingers and the muscles of the medial forearm. It is responsible for wrist flexion and, along with the radial nerve, enables the thumb to oppose the other four fingers. A normally perceived pinprick over the plantar surface of the distal fifth finger generally indicates an intact ulnar nerve (see Figure 6-7).
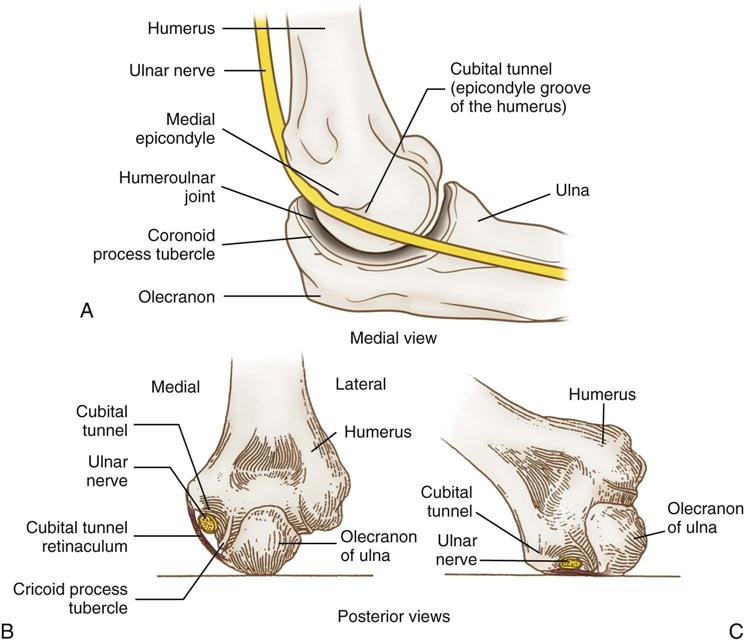
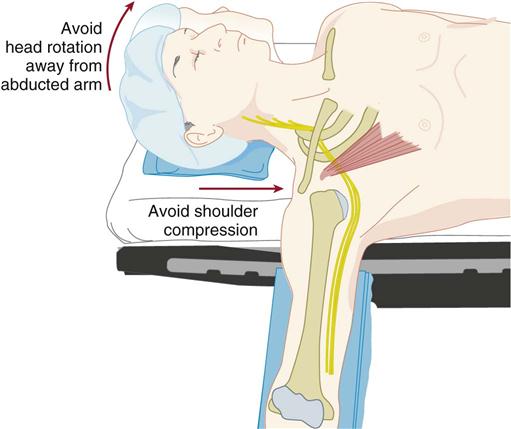
The most common OR-related nerve injuries are to the ulnar nerve and brachial plexus. There is a good reason for this. As the ulnar nerve circles behind the elbow, it lies superficially in the shallow cubital tunnel of the humerus, where it is subject to pressure and to stretching from flexion of the elbow (Figure 6-8). The brachial plexus resides in the shoulder, which is subject to abduction, manipulation, and sometimes pressure, depending on position. The use of shoulder braces, to reduce sliding while in Trendelenburg position, should be avoided because braces can compress proximal nerve roots and stretch the brachial plexus. Arm abduction on armboards should be limited to 90 degrees or less. Avoid excessive head rotation, especially away from the abducted arm because doing so can stretch and compress the nerves between the clavicle and the first rib (Figure 6-9).
The brachial plexus can also suffer injury by the separation of the sternum during open cardiac procedures that require a median sternotomy. When splitting the sternum, a retractor is used to separate it, allowing access to the heart. This separation forces the ribs to move laterally. If internal mammary artery dissection occurs as part of the procedure, there is additional asymmetric retraction of the rib cage. The first rib compresses the brachial plexus nerve bundles and, in particular, the nerve cord that emerges into the ulnar nerve. If brachial plexus and ulnar neuropathies develop as a result, they generally are not preventable by proper positioning of the patient’s arm (Warner, 2009). Improper arm positioning, however, may worsen the insult of the injury.
Isolated ulnar nerve injuries arise primarily from pressure on the vulnerable location of that nerve. The ulnar nerve becomes superficial as it runs behind the elbow, nesting in the epicondyle groove of the humerus, which makes up the cubital tunnel. Ulnar nerve injuries occur primarily in male patients. Warner (2009) offers three anatomic differences between the elbows of men and women, which probably account for this gender anomaly:
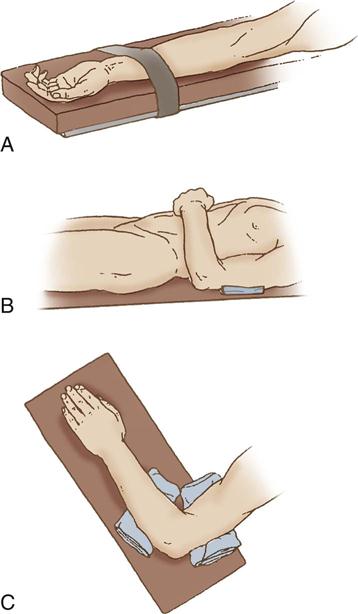
The main goal in protecting the ulnar nerve is to eliminate pressure on it. Pressure on the medial aspect of the elbow may compress the ulnar nerve (see Figure 6-8, C). Figure 6-10 illustrates ways to prevent this pressure. Supination (palms up) of the forearms on an armboard (see Figure 6-10, A) places the olecranon of the elbow on the flat surface instead of the cubital tunnel, which contains the ulnar nerve. When the arm of a supine patient rests on the trunk, direct trauma can occur to the ulnar nerve as the weight of the arm presses the flexed elbow against the surface of the bed. Padding placed under the upper arm, proximal to the elbow, leaves a free space under the elbow, eliminating pressure on the nerve (see Figure 6-10, B). When a patient is prone (abdomen facing down) with arms pronated on armboards, the ulnar nerve is vulnerable to pressure from the elbow. Padding placed proximal and distal to the elbow frees the nerve from pressure (see Figure 6-10, C).
Ulnar neuropathy symptoms may manifest in the immediate postoperative period. Sometimes symptoms are delayed until 2 to 7 days after surgery, however, which makes it difficult to isolate the exact event that triggered the complication. The postoperative course cannot be completely ruled out as a possible cause or contributing factor. Some patients are forced to lie on their backs for prolonged periods postoperatively because of devices such as endotracheal tubes and abdominal drains. Often they rest with elbows flexed and hands on their chest. This may place pressure on that superficial section of the ulnar nerve under the elbow, which may in turn cause or contribute to injury to the nerve during the postoperative period.
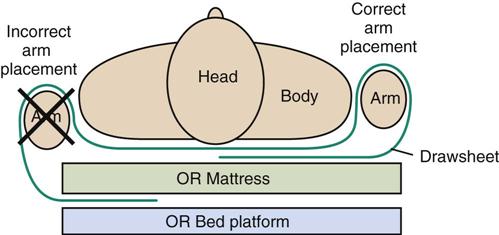
Many OR-induced peripheral upper extremity nerve injuries can be avoided by properly securing the arms when they are tucked at the patient’s side. The arms should be tucked in a way that prevents them from sliding down the side of the OR bed and contacting the bed edge or rigid bed attachments. An effective technique to prevent arm slippage during surgery is to wrap the drawsheet smoothly around the arm and then tuck the drawsheet under the patient’s body instead of under the mattress (Figure 6-11). This technique is useful in the supine and prone positions. It is best accomplished with an assistant on the other side of the OR bed, who rolls the patient’s body over enough to tuck the drawsheet underneath it. Care should be taken to ensure that the drawsheet is not tucked too tightly as to cause pressure to the arms. A tight fold pressing on the elbow could cause an isolated ulnar nerve injury.
Complete and thorough documentation of intraoperative measures taken to protect vulnerable nerves should include the following:
• How the arms were secured and by whom
• Times and type of repositioning or passive range of motion done
The upper extremities should be evaluated postoperatively. Any changes from the preoperative condition should be verbally reported and documented.
Lower Extremity Neuropathies.
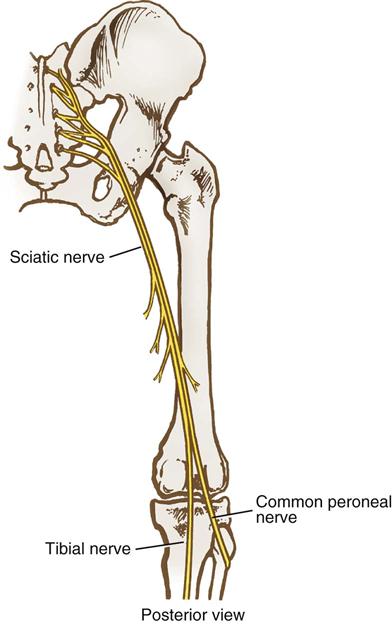
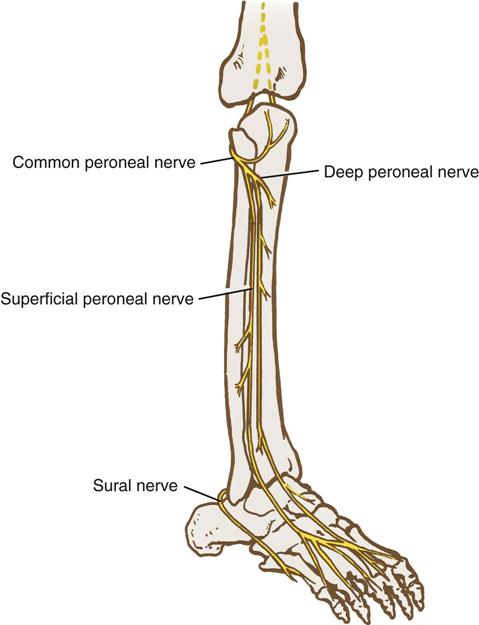
Lower extremity neuropathies result most frequently from prolonged lithotomy positioning and tend to manifest symptoms within hours after surgery. The common peroneal, sciatic, and femoral nerves are most frequently implicated.
Intraoperative risk factors include the length of time in lithotomy, high or exaggerated lithotomy, and positioning the extremities beyond their comfortable range of motion when awake. Different types of stirrups vary in the degree to which they control hip flexion. Hip extension should be limited to the minimum amount required for adequate access to the surgical site.
The common peroneal nerve branches from the sciatic nerve behind the knee and becomes superficial as it wraps around the lateral head of the fibula (Figures 6-12, 6-13, and 6-14). At this level, it is vulnerable to direct compression by stirrup bars. This risk may be increased in extremely thin patients who have minimal overlying tissue in this area. It is important to ensure that the lateral head of the fibula does not rest against stirrup bars or any other rigid surface. Compressive leg wraps (i.e., intermittent pneumatic compression devices, graduated compression stockings) also can put pressure on this nerve if the wrapping is too tight in this area. In addition, pressure behind the knee can compress the common peroneal and tibial nerves where they run through the popliteal fossa, so only soft padding or pillows should be used for knee support. The patient’s ability to dorsiflex the great toe (point it upward) indicates that the common peroneal nerve is intact.
The sciatic nerve originates from the L4-S3 spinal nerve roots and travels down the buttock and posterior thigh before it divides into the common peroneal and tibial nerves (see Figure 6-12). The stretching that occurs during hyperflexion of the hip may injure it. A patient who has had a total hip replacement may have some preexisting sciatic nerve damage as a result of the excessive rotation of the femur that is part of that operative procedure. Additionally, patients with a history of hip trauma who have required surgery such as open reduction and internal fixation may be at increased risk for sciatic neuropathy because of the potential presence of scar tissue around the nerve area. Because the branches of the sciatic nerve run from the thigh to the hamstring, the patient’s ability to flex the thigh generally indicates an intact sciatic nerve.
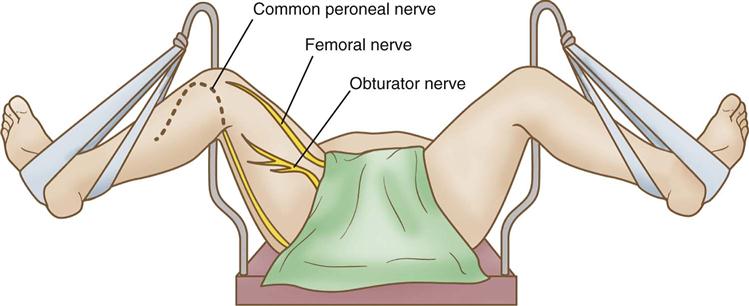
The femoral nerve arises from the L2-L4 spinal nerve roots and runs through the medial thigh. Injury to the deep femoral nerve may occur more as a result of inappropriate placement of abdominal pelvic retractors and vaginal retractors in the pelvic cavity than by virtue of inappropriate positioning (Cassorla and Lee, 2010). The femoral nerve and obturator nerve can be subjected to excessive stretching and injury, however, as a result of inappropriate lithotomy positioning (see Figure 6-14). The patient’s ability to flex the thigh to the trunk generally indicates an intact femoral nerve.
The obturator nerve originates from the L2-L4 nerve roots. It runs through the obturator foramen of the ischium to innervate the adductor group of muscles in the inner thigh (see Figure 6-14). It can be subjected to excessive stretching when the patient is in lithotomy position. Scrubbed personnel may lean against the inner thigh while holding vaginal or anal retractors, causing additional stretching and compression of the nerve. The patient’s ability to adduct the leg generally means the obturator nerve is intact.
The tibial nerve branches from the sciatic nerve behind the knee and runs along the posterior tibia to the foot (see Figure 6-12). From there it branches into the medial and lateral plantar nerves. The patient’s normal sensation at the plantar surface of the foot and ability to curl the toes downward generally indicate an intact tibial nerve.
The perioperative nurse should document measures taken to protect the lower extremities from injury, such as:
• Location and type of padding used (i.e., pillow to support knees or calves, padding under heels)
• Type of stirrups used; names of persons placing legs in stirrups
• Type of lithotomy position (low, standard, high, or exaggerated)
• Presence of distal pulses, color, and temperature of legs and feet
• Use of intermittent pneumatic compression devices or graduated compression stockings
The lower extremities should be evaluated postoperatively. Any changes from the preoperative condition should be verbally reported and documented.
The use of intraoperative neurophysiologic monitoring is demonstrably an effective method to prevent peripheral nerve injuries in the upper and lower extremities during procedures in which there is otherwise a high risk of such injuries. A common method of monitoring in the OR is somatosensory-evoked potential (SSEP). Its goal is to establish a baseline of the patient’s normal nerve function along selected pathways and then to monitor those pathways at continual intervals during the procedure. Any emerging insult to these nerve structures or pathways will appear and corrective measures taken before permanent nerve injury occurs (Chung et al, 2009).
The Association of periOperatve Registered Nurses (AORN, 2013) offers specific recommendations to prevent nerve injuries during surgery. They are listed in the Evidence for Practice box.
Vascular System
General anesthesia causes peripheral vessels to dilate by depressing the sympathetic nervous system. This dilation decreases overall blood pressure because blood pools in dependent areas of the body. In general anesthesia these effects are systemic, whereas in regional anesthesia, such as spinal and epidural, these effects are more limited to the areas anesthetized.
Position changes affect where blood pooling occurs. Muscle tone and peripheral vascular resistance are no longer as effective in counteracting the forces of gravity on blood pooling. Blood pools to whatever part of the body is lowest. If the head of the OR bed is raised, the lower torso has increased blood volume, and the upper torso becomes more compensated. Hypovolemia and cardiovascular disease may compromise the patient’s status further.
The anesthesia provider can treat some of the hypotensive effects of positioning through pharmacologic agents and increased IV infusion. Vital signs, IV intake, and urinary output need to be monitored closely. Position changes may need to be delayed until blood pressure stabilizes.
Deep vein thrombosis (DVT) is a serious complication of surgical positioning. Compression to deep and peripheral vessels (e.g., resulting from tight safety strap or wrist restraints) may predispose the patient to venous thrombosis. When the legs are in a dependent position (i.e., legs lowered during knee arthroscopy, sitting position, reverse Trendelenburg), venous return slows and thrombosis can occur (Pannucci et al, 2011). Thrombosis also can occur as a result of hyperabduction of the arms beyond 90 degrees. Subclavian and axillary vessels pass through the brachial plexus, and vessel constriction can occur by compression between the clavicle and the first rib. Radial pulses require monitoring whenever arms are extended to ensure that the radial pulse is not obliterated. Graduated compression stockings or intermittent pneumatic compression devices can reduce this risk.
Vascular assessment of upper and lower extremities should occur preoperatively. Document measures taken to reduce assessed risks and evaluate vascular status intraoperatively and postoperatively. Any changes from the preoperative condition require verbal reports and documentation.
Acute compartment syndrome is a serious complication that may arise from excessive constriction to, or improper positioning of, extremities. It is characterized by ischemia, hypoxic edema, and elevated tissue pressure within the fascial components of the extremity, leading to further damage to the muscles and nerves. This process occurs at a cellular level, and distal pulses and capillary refill may initially appear normal; then, however, the process may rapidly escalate to a dangerous condition. The syndrome may progress to rhabdomyolysis with resulting renal damage, permanent nerve damage, loss of limb, or even death. The most effective means to end this syndrome is a decompressive fasciotomy.
Respiratory System
Positioning can compromise the respiratory system. In almost all types of positions, except semi-Fowler, sitting, and reverse Trendelenburg, the abdominal viscera shift upward toward the diaphragm. Subsequently, the diaphragm shifts upward and outward such that it contributes only about two thirds of the ventilatory force and significantly reduces tidal volume. Obese or pregnant patients or those who have pulmonary disease have additional respiratory compromise in these positions. Any patient who experiences dyspnea should have the head of the bed elevated during transport and during local, regional, or spinal anesthesia if this does not interfere with surgical access. During general anesthesia the anesthesia provider normally ventilates these patients mechanically.
External chest movement during surgery needs to be as unrestricted as possible to avoid further reduction in tidal volume. Avoid placement of arms across the chest because arms resting on the chest not only restrict chest expansion, but also add pressure on the ulnar nerve at the elbow area. Some positions (e.g., lateral, fracture bed) require the placement of straps or tape around the chest area to secure the patient to the OR bed without obstructing the surgical site. Such strapping or taping requires efforts to ensure against excessive tightness. These positions are not maintained any longer than necessary. Anesthesia personnel closely monitor the respiratory status of these patients.
Positioning also affects the portions of the lungs perfused with blood. As blood pumps into the lungs, the areas of the lung that are most dependent have a greater pulmonary arterial pressure as the blood pools. The greater the pressure, the greater is the perfusion. The alveoli in the bases of the lungs are more compliant; ventilation is generally highest in those areas. An even distribution of ventilation and perfusion (ventilation-perfusion ratio) is needed for efficient gas exchange. If the position causes a pathologically compromised area of the lung to be dominant and more perfused, the balance between perfusion and ventilation may be disrupted and respiratory status may diminish. The rest of the lung fields may or may not have the capacity to compensate. Oxygen therapy or mechanical ventilation may help compensate, but the amount of time a patient can tolerate a certain position and maintain adequate gas exchange may be limited.
Monitoring of respiratory status should always include pulse oximetry. In some cases, intraoperative arterial blood gas monitoring is also necessary.
Obesity
Obesity is an important consideration in the perioperative environment because its incidence has been steadily rising over the past decades. The upward trend exists among children, teens, and adults. The degree of obesity is based on body mass index (BMI). Obesity and morbid obesity are considered to be BMI of ≥30 kg/m2 or ≥40 kg/m2 (weight)/m2(height), respectively (Ogunnaike and Whitten, 2009). Morbid obesity is a complex medical disorder that frequently coexists with numerous other medical problems. These include arthritis and degenerative joint disease, sleep apnea (Ambulatory Surgery Considerations), hypertension, type 2 diabetes, and esophageal reflux. The incidence of these conditions increases with the age of the patient and the duration of severe obesity (Richards, 2012). Morbid obesity is associated with some considerable perioperative risks (Patient Safety). Obese patients have a diminished respiratory reserve and become distressed quickly and easily when lying flat in supine. Trendelenburg is even more poorly tolerated and should be avoided until the patient is intubated and mechanical ventilation can assist with breathing (Ide et al, 2008). With excess fat distribution around the patient’s back, neck and shoulders, positioning interventions are necessary to gain control of the patient’s airway in the supine position. This is done by what has been called “ramping,” that is, building a ramp of cushioning materials (e.g., pillows, linens, foam wedges) in order to elevate the head, neck, and shoulders above the chest into alignment with the airway. To create the correct plane, an imaginary horizontal line can be drawn between the earlobe and the sternum as noted in Figure 6-15. This ramped position enables the anesthesia provider to gain control of the patient’s airway by aligning the oral, laryngeal and pharyngeal axes. It has also been called the head-elevated laryngoscopy position (HELP) because it facilitates laryngoscopy and intubation (Ogunnaike and Whitten, 2009).