Portal Vein Resection and Reconstruction
Steven J. Hughes
Kevin E. Behrns
DEFINITION
Borderline resectable pancreatic cancer is defined by tumor involvement of mesentericoportal venous axis.1
En bloc portal vein (PV) resection with immediate reconstruction is now routinely performed by most high-volume pancreatic surgery centers. Large clinical series have demonstrated that this additional operative complexity does not result in increased morbidity.2,3
Arterial resection and reconstruction is not indicated because existing data do not show a favorable benefit-to-risk ratio.
PATIENT HISTORY AND PHYSICAL FINDINGS
Most borderline resectable patients will ultimately be staged with N1 disease.4 Thus, these patients have a 5-year survival that is very close to the operative mortality.
Previous central venous access or venothromboembolic events may be a contraindication to venous reconstruction because of venous hypertension.
A history of hypercoagulability must be considered as an additional risk and factored into consideration for surgical therapy and potential for postoperative anticoagulation.
Lower extremity edema is a contraindication for use of the deep femoral vein as conduit.
IMAGING AND OTHER DIAGNOSTIC STUDIES
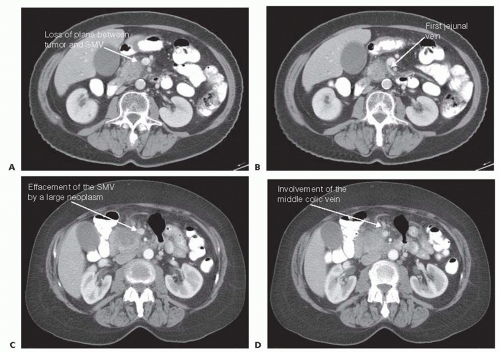
A high-quality, multidetector, thin-slice, triphasic computed tomography (CT) scan is essential (FIG 1). Pay particular attention to the PV phase; loss of the fat plane for greater than 180 degrees or narrowing of the mesentericoportal complex are predictive of PV involvement by neoplasm.5
Obtain bilateral duplex ultrasound studies of the deep femoral and jugular veins. Surface mapping of deep femoral vein assists in harvesting the conduit but is not required for success.
PREOPERATIVE PLANNING
Key to success is the accurate, preoperative determination that an interposition graft will be required.
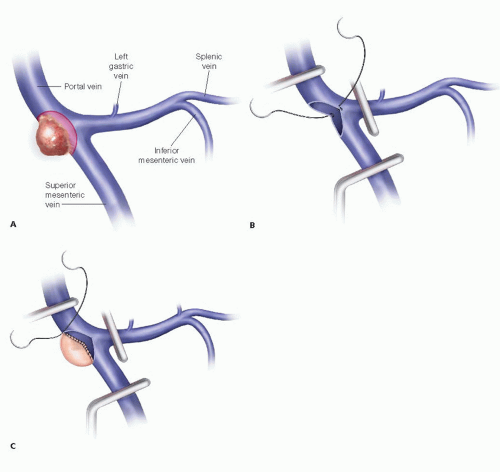
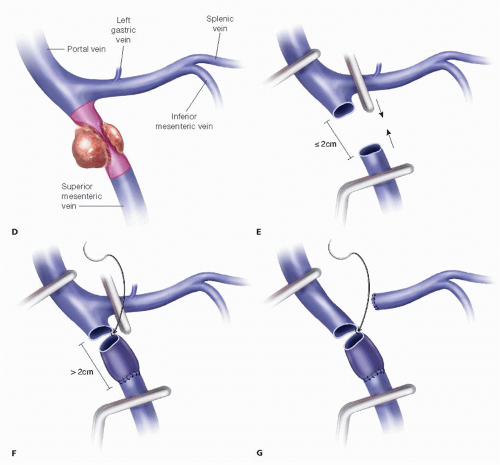
If the vein is narrowed, plan for a circumferential excision with primary repair versus interposition graft. Using the CT scan, estimate the length of the PV involved and the resulting defect requiring reconstruction (FIG 2).
A defect measuring less than 2 cm can usually be repaired primarily.
A defect 2 to 4 cm typically can be reconstructed using an interposition graft.
Defects measuring more than 4 cm will result in a longsegment conduit; the risk of graft thrombosis is proportional to the length, and longer segment reconstructions may preclude surgical therapy.
Tumor involvement with respect to the splenic vein (SV) and superior mesenteric vein (SMV) confluence must be considered.
If the lateral wall is involved, a primary transverse repair or patch repair is ideal.
If a circumferential vein resection is required and a primary end-to-end anastomosis or interposition graft planned, most experienced surgeons do not reinsert the SV—this significantly increases the complexity of the procedure. Rather, the SV is ligated proximally. The risk of sinister portal hypertension leading to symptoms is acceptably low.
Consider initiating or continuing a daily enteric-coated aspirin (81 mg) through the perioperative period.
Have a bovine pericardium patch available.
Most centers have abandoned the use of cadaveric vein grafts.
Strongly consider a neoadjuvant approach for all patients with borderline resectable disease. An R1 resection confers no survival benefit over a nonsurgical, palliative therapeutic approach.
Neoadjuvant therapy should be offered to all patients in whom there is a loss of the fat plane around the superior mesenteric artery (SMA).
TECHNIQUES
POSITIONING
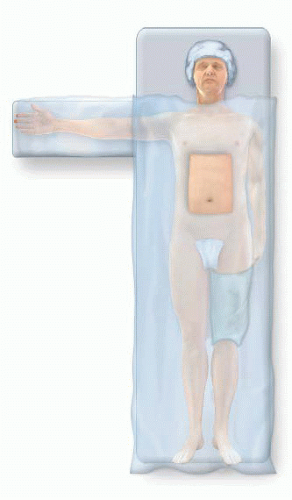
Tuck the left arm. This will provide better access to the left neck should the jugular vein need to be procured as a graft (FIG 3).
Place either an upper or lower body active warming device; this decision is driven by the planned source of conduit.
Prepare the patient’s skin. Then, isolate the left neck or left thigh and abdominal operative fields with sterile towels (FIG 4). Ensure that these towels create a sterile field between these isolated sites. Use ¾ sterile drapes as indicated to achieve this objective. The authors support the use of iodine-containing, self-adhesive barriers to secure these fields under the additional overlying draping and reduce the risk of surgical site infections.
To minimize the risk of hypothermia, drape the patient such that only the abdominal operative field is exposed. This drape will be cut to expose the graft harvest site when indicated.
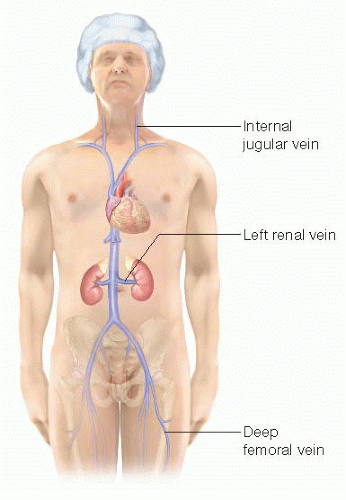 FIG 3 • Autologous sources of interposition vein grafts include the jugular, left renal, and deep femoral veins. |
INCISION AND EXPOSURE
Either a midline or bilateral subcostal incision can provide adequate exposure. In addition to exposing the upper abdomen, the incision must allow for complete mobilization of the right colon and retroperitoneal attachments of the small bowel mesentery.
DISSECTION
The dissection proceeds for the most part as presented in detail in Part 3, Chapter 33 but may include limiting particular portions of the dissection; most importantly, the uncinate process/SMA dissection is performed prior to dissection of the SMV/PV.
Perform a complete Cattell-Braasch maneuver, fully mobilizing the retroperitoneal attachments of the cecum, right colon, and small bowel mesentery to the left of the aorta and up to the third portion of the duodenum.
As part of the Kocher maneuver, establish a plane between the third portion of the duodenum and the aorta and extend this dissection cephalad to the origin of the SMA (FIG 5).
Mobilize the ligament of Treitz and derotate the small bowel such that the cecum is in the left upper quadrant.
These maneuvers will provide excellent exposure and mobility of the mesentery to facilitate primary reconstruction without undue tension following mesentericoportal resections up to 2 to 3 cm in length.
This mobilization will also expose the anterior aspect of the left renal vein—a potential source of reconstruction conduit.
Open the lesser space and expose the pancreatic head. Assess the root of the transverse mesocolon and bimanually palpate the lesion. Also, palpate the course of the SMA with respect to the palpable lesion.
Proceed with the dissection of the inferior and superior borders of the pancreas, identifying the anterior borders of the SMV and PV proximal and distal to the area of tumor involvement, respectively.
By palpation and visualization, identify the relationship of the tumor with respect to the SV/SMV confluence.
Establish the plane between the root of the mesentery and the third and fourth portions of the duodenum and uncinate process.
Determine resectability with respect to involvement of the SMV/PV and the extent of resection required (FIG 2).
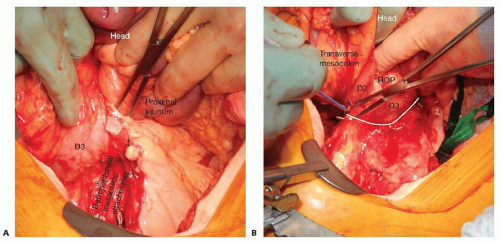
FIG 5 • A. Completely mobilize the right colon and the retroperitoneal attachments of the small bowel. B. Extend the dissection by mobilizing the retroperitoneal attachments of the duodenum and pancreatic head to the origin of the SMA (white line represents plane of dissection). D2, second portion of the duodenum. D3, third portion of the duodenum. HOP, head of pancreas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access