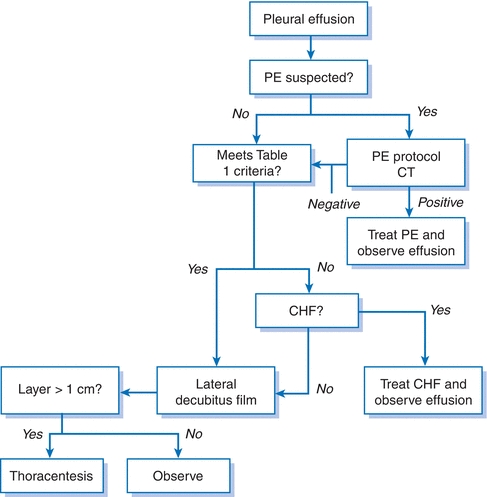
Figure 19-1 Evaluation of the unknown pleural effusion. CHF, congestive heart failure; CT, computed tomography; PE, pulmonary embolism.
Diagnostic Procedures
Thoracentesis
- Once a pleural effusion has been identified, the clinician must decide whether to sample the pleural fluid for either diagnostic or therapeutic benefit or both. Table 19-1 shows indications for thoracentesis, while Figure 19-1 is a schematic for the evaluation of an unknown effusion.
- If a patient has significant symptoms of dyspnea, cough, pain, or a supplemental oxygen requirement, a therapeutic thoracentesis is warranted.
- Thoracentesis can be performed safely, in the absence of disorders of hemostasis, on effusions that demonstrate a >10 mm on a lateral decubitus film.
- Loculated effusions can be localized with ultrasonography or CT scan.
- The most common serious complications of thoracentesis are pneumothorax, bleeding, and introduction of infection into the pleural space.
- Proper technique and sonographic guidance minimize the risk of complications.
- Pleural fluid appearance may be helpful in diagnostic and therapeutic considerations.
- Red-tinged or serosanguineous pleural effusions indicate the presence of blood. This is either due to the procedure (and therefore should clear with continued aspiration) or from the primary disorder (commonly malignancy, PE, or trauma).
- The presence of gross blood should lead to the measurement of a pleural fluid hematocrit. Hemothorax is defined as a pleural fluid blood hematocrit ratio of more than 0.5, and chest tube drainage should be implemented.
- Malodorous fluid or frank pus is consistent with an empyema, and tube thoracostomy should occur immediately.
- Turbid or milky fluid should prompt evaluation for chylothorax.
- Red-tinged or serosanguineous pleural effusions indicate the presence of blood. This is either due to the procedure (and therefore should clear with continued aspiration) or from the primary disorder (commonly malignancy, PE, or trauma).
- The most important aspect of pleural fluid analysis is the laboratory evaluation, allowing the designation of a pleural effusion as either transudate or exudate using Light criteria (Table 19-2) or Heffner criteria (Table 19-3).1–3 Of note, in patients who have an exudative effusion by chemical criteria, but high clinical suspicion for heart, liver, or kidney disease, then a serum to pleural fluid albumin gradient should be checked. A gradient of >1.2 g/dL suggests that the pleural fluid is likely due to congestive heart failure (CHF), liver, or kidney disease.4
- The most frequently used criteria for defining pleural fluid as either exudate or transudate is Light criteria.1 These criteria have a 97.9% sensitivity for detecting an exudative effusion.5 At the time of thoracentesis, a simultaneous measurement of serum lactate dehydrogenase (LDH) and protein must be sampled to properly use the criteria.
- Heffner criteria have a similar sensitivity to Light criteria (98.4%) and do not require simultaneous blood work for interpretation.3
- The most frequently used criteria for defining pleural fluid as either exudate or transudate is Light criteria.1 These criteria have a 97.9% sensitivity for detecting an exudative effusion.5 At the time of thoracentesis, a simultaneous measurement of serum lactate dehydrogenase (LDH) and protein must be sampled to properly use the criteria.
- High cell counts are more typically seen in exudative effusions; however, this is not a component of Light criteria.
- A high neutrophil count is suggestive of an infectious process, especially bacterial, and should prompt consideration of an empyema.6
- Eosinophilia (>10% of total nucleated cell count) is suggestive of air or blood in the pleural space.7 If neither of these is present, consideration should be given to fungal or parasitic infection, drug-induced disease, PE, asbestos-related disease, and Churg-Strauss syndrome.8
- Lymphocytosis (>50% of the total nucleated cell count) is suggestive of malignancy or tuberculosis.6
- Mesothelial cells, when present, argue against the diagnosis of tuberculosis.
- Plasma cells in abundance suggest multiple myeloma.
- A high neutrophil count is suggestive of an infectious process, especially bacterial, and should prompt consideration of an empyema.6
- Routine stains should be obtained to quickly determine if an effusion is infected and to direct antibiotic therapy if indicated (Table 19-4). Staining for acid-fast bacilli and culture for tuberculosis are performed when clinically indicated.
- A glucose concentration <60 mg/dL is probably due to tuberculosis, malignancy, rheumatoid arthritis, or a parapneumonic effusion.9–11 For parapneumonic effusions, with a glucose <60 mg/dL, tube thoracostomy should be considered (Table 19-4).
- Pleural fluid with a low pH usually corresponds to a low glucose and a high LDH; otherwise, the low pH may be due to poor sample collection technique (proper pH testing on pleural fluid involves anaerobic collection in a heparinized syringe and stored on ice).
- A pH of <7.3 is seen with empyema, tuberculosis, malignancy, collagen vascular disease, or esophageal rupture.
- For parapneumonic effusions with a pH < 7.2, tube thoracostomy should be considered (Table 19-4).8,12
- A pH of <7.3 is seen with empyema, tuberculosis, malignancy, collagen vascular disease, or esophageal rupture.
- Cytology is positive in approximately 60% of malignant effusions.13 Priming the fluid collection bag with unfractionated heparin may increase the yield. Of note, the volume of pleural fluid analyzed does not impact the yield of cytologic diagnosis.14
- An elevation in amylase suggests pancreatic disease, malignancy, or esophageal rupture but should not be routinely measured unless there is a clinical suspicion.15 Malignancy and esophageal rupture have salivary amylase elevations and not pancreatic amylase elevations.
- Turbid or milky fluid should prompt an investigation for chylothorax. The fluid should be centrifuged. If the cloudiness clears, then the appearance was merely secondary to cells and debris. If the supernatant does not clear and instead remains turbid, then pleural lipids should be checked. Elevation of triglycerides (>110 mg/dL) suggests that a chylothorax is present,2,8 usually due to a disruption of the thoracic duct from trauma, surgery, or malignancy (e.g., lymphoma). Chylomicrons in the pleural fluid will confirm this.
TABLE 19-2 Light Criteria for Definition of an Exudate

TABLE 19-3 Heffner Criteria for Definition of an Exudate
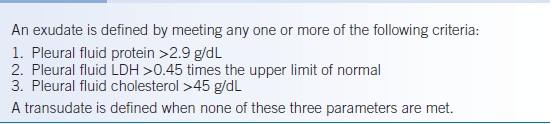
TABLE 19-4 Indication for Tube Thoracostomy in Parapneumonic Effusions

Other Diagnostic Procedures
- When there is clinical suspicion for a certain diagnosis, other invasive procedures may be useful.
- Closed pleural biopsy typically adds little to the diagnostic yield of thoracentesis, except in the diagnosis of tuberculosis. For tuberculous effusions, pleural fluid cultures alone are positive in only 20% to 25% of cases. However, the combination of pleural fluid studies and pleural biopsy (demonstrating granulomata or organisms) is 90% sensitive in establishing TB as the etiology of the effusion.
- Diagnostic thoracoscopy has largely replaced closed pleural biopsy. Thoracoscopy allows visually directed biopsies, thus increasing the diagnostic yield for malignancy, while maintaining the high diagnostic yield for TB.
Differential Diagnosis
- Transudative pleural effusions usually have low protein and LDH. The glucose level is usually similar to the serum level, and the pH is generally higher than blood pH. Most transudates are clear, straw colored, nonviscous, and odorless. White blood cell (WBC) count is usually <100 cells/HPF, and the RBC count is usually <10,000 cells/HPF. Transudates should lead to further evaluation of the heart, liver, and kidney with therapy directed accordingly.
- Exudative pleural effusions usually have high-protein or LDH values and meet one of Light criteria as described above. Diagnosis of the etiology of the pleural fluid should proceed with a careful history and physical followed by pleural fluid analysis. There is a broad differential for exudative pleural effusions, and once a diagnosis is determined, therapy should be directed toward the cause.
- Parapneumonic effusions are exudates that develop secondary to pulmonary infections.
- Patients with pneumonia and an effusion should undergo rapid diagnostic testing because an infected pleural space (empyema) needs to be treated without delay.
- In an uncomplicated parapneumonic effusion, bacterial infection in the pleural space is presumed to be absent, pH > 7.2, and glucose >60 mg/dL.
- In complicated parapneumonic effusions, bacterial invasion of the pleural space is assumed to be present, pH < 7.2, glucose <60 mg/dL, and/or Gram stain and/or culture positive. Empyema is any uncomplicated parapneumonic effusion that appears grossly purulent.
- Complicated parapneumonic effusions and empyema should be managed with chest tube drainage when indicated based on the size, presence of loculations, gross appearance of the fluid, or biochemical analysis of the pleural fluid (Table 19-4).12
- Antibiotics should be administered broadly and then narrowed as directed by culture data.
- Multiple chest tubes are sometimes required to adequately drain the pleural space.
- Failure to adequately and quickly drain the pleural space can lead to organization of the pleural fluid and formation of a thick pleural “rind,” which may necessitate surgical removal (known as decortication).
- Patients with pneumonia and an effusion should undergo rapid diagnostic testing because an infected pleural space (empyema) needs to be treated without delay.
- Malignant pleural effusions arise from tumor involvement of the pleura or mediastinum. In addition, patients with cancer are at increased risk of pleural effusions from other secondary causes such as PE, postobstructive pneumonia, chylothorax, and drug and radiation reactions. It may be appropriate for some patients with stable effusions without significant symptoms to avoid further invasive procedures and observe only.
- Parapneumonic effusions are exudates that develop secondary to pulmonary infections.
TREATMENT
- Therapeutic thoracentesis may improve patient comfort and relieve dyspnea.
- Repeated thoracenteses are reasonable if they achieve symptomatic relief and if fluid reaccumulation is slow.
- The incidence of reexpansion pulmonary edema is approximately 1% and is related to changes in pleural pressure more so than the absolute volume of pleural fluid that is removed.16
- Unfortunately, 95% of malignant effusions recur, and the median time to recurrence is <1 week.
- Repeated thoracenteses are reasonable if they achieve symptomatic relief and if fluid reaccumulation is slow.
- Pleurodesis is an effective procedure indicated for a recurrent pleural effusion in a patient whose symptoms were relieved with initial drainage but has rapid reaccumulation.
- Chemicals used for pleurodesis include talc, doxycycline or minocycline, and bleomycin (considered less effective and more expensive).
- Chemical sclerosant is instilled into the pleural space to promote fusion of the visceral and parietal pleura (pleurodesis).
- Systemic analgesics and lidocaine added to the sclerosing agent should be used to reduce the significant discomfort associated with this procedure.17
- If chest tube drainage remains high (>100 mL/day) >2 days after the initial pleurodesis, a second dose of sclerosing agent can be administered.
- Chemicals used for pleurodesis include talc, doxycycline or minocycline, and bleomycin (considered less effective and more expensive).
- A chronic indwelling pleural catheter (e.g., PleurX catheter) can provide good symptomatic control of an effusion via intermittent patient-controlled drainage.
- The PleurX catheter is better at controlling symptoms than doxycycline pleurodesis.18
- Furthermore, repeated drainage leads to pleurodesis in roughly 50% of patients, allowing the catheter to be removed.
- Chronic indwelling catheters can be used for palliation with trapped lung compared when chemical pleurodesis is ineffective due to incomplete apposition of the visceral and parietal pleura.
- The PleurX catheter is better at controlling symptoms than doxycycline pleurodesis.18
- Pleurectomy or mechanical pleural abrasion of the pleural lining can promote pleurodesis. This requires thoracotomy and should be reserved for patients with a good prognosis who have had ineffective pleurodesis by other means.
- Chemotherapy and mediastinal radiotherapy may control effusions in responsive tumors, such as lymphoma or small cell bronchogenic carcinoma.
Solitary Pulmonary Nodule
GENERAL PRINCIPLES
- The solitary pulmonary nodule (SPN) is defined as a ≤3 cm isolated, spherical, well-circumscribed lesion completely surrounded by aerated lung without associated atelectasis, hilar enlargement, or pleural effusion.19,20
- A lesion >3 cm is referred to as a pulmonary mass, and the probability of malignancy is much higher.19,20 Some authorities also distinguish subcentimeter nodules as <8 to 10 mm, which are much less likely to be malignant.19
- The large majority of SPNs are discovered incidentally on plain CXR or chest CT obtained for other reasons.19
- The prevalence of SPNs is highly dependent on the characteristics of the population studied (e.g., age, smoking status) and the technique used (e.g., CXR or CT). It has been reported to range from 0.2% to 7% for CXRs and 8% to 51% for CT.19,21–23 Some SPNs detected on CXR will be false positives.
- Importantly, long-term survival is dramatically better after resection of a malignant SPN compared to that for advanced lung cancer.
- Screening high-risk patients with low-dose CT scans significantly decreases lung cancer–attributed mortality in this patient population.24
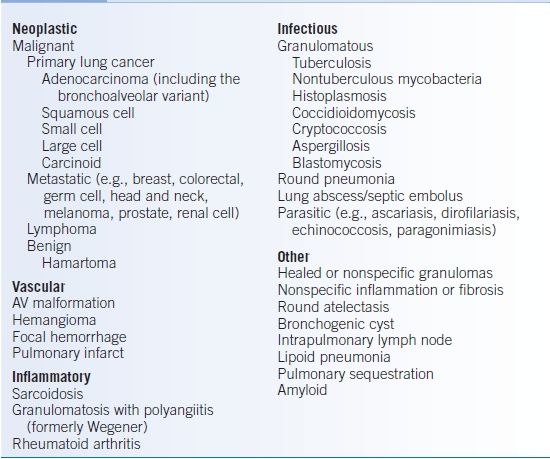
- The rate of malignancy in patients with SPNs varies greatly depending on study populations and methods of detection used. Characteristics that increase the risk of malignancy will be discussed below. Table 19-5 outlines a differential diagnosis for SPN.
TABLE 19-5 Partial Differential Diagnosis of the SPN
DIAGNOSIS
Clinical Presentation
- The vast majority of patients with a SPN will be asymptomatic with regard to the nodule itself. Age, smoking status, history of extrathoracic cancer, and history of prior lung cancer are perhaps the most important historical features that increase the likelihood that an SPN is malignant.19,20,

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


