Pleomorphic Adenoma
Brenda L. Nelson, DDS, MS
Key Facts
Terminology
Synonym: Benign mixed tumor
Benign epithelial tumor that shows epithelial, myoepithelial, and mesenchymal differentiation
Clinical Issues
Most common neoplasm of salivary gland origin
Parotid gland most common site
Slow growing
Minor salivary glands 2nd most frequent site affected
Macroscopic Features
Recurrent tumors are generally multinodular
Irregular mass
Parotid gland
Variably thick capsule
Rarely unencapsulated
Minor glands
Poorly developed to absent capsule
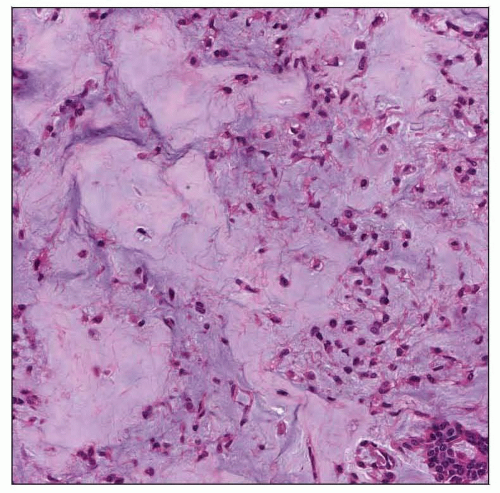
Microscopic Pathology
Innumerable cytologic and architectural patterns
Epithelial tissue
Mesenchymal-like tissue
Top Differential Diagnoses
Myoepithelioma
Basal cell adenoma
Adenoid cystic carcinoma
Polymorphous low-grade adenocarcinoma
Carcinoma ex-pleomorphic adenoma
Diagnostic Checklist
No two look alike
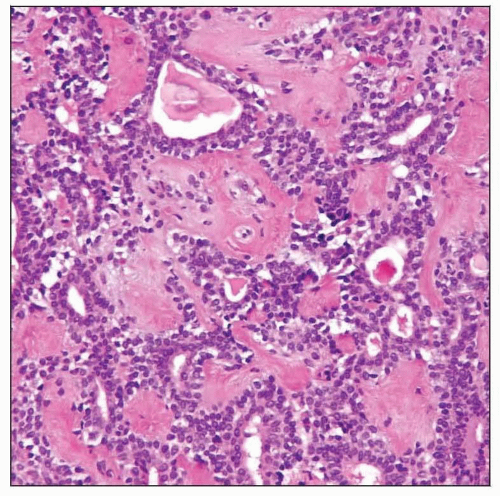 This tumor shows characteristic areas of tubular and ductal structures with a background of hyaline stroma. Pleomorphic adenomas show amazing microscopic diversity. |
TERMINOLOGY
Abbreviations
Pleomorphic adenoma (PA)
Synonyms
Benign mixed tumor (BMT)
Mixed tumor
Chondroid syringoma
Only used if skin/dermis based primary
Definitions
Benign epithelial tumor that shows both epithelial and modified myoepithelial elements mixed with mesenchymal myxoid, mucoid, or chondroid appearing material
Significant architectural diversity rather than cytologic pleomorphism
CLINICAL ISSUES
Epidemiology
Incidence
Most common neoplasm of salivary gland origin
45-76% of all salivary gland neoplasms
Comprises about 75% of all major salivary gland neoplasms
Comprises about 40% of all minor salivary gland neoplasms
Approximately 3/100,000 population
Age
Wide age range
Peak in 4th-6th decade
Most common benign salivary gland tumor in children
Gender
Female > Male (slightly) in adults
Male > Female in children (< 18 years)
Site
Parotid gland most common site (approximately 80%)
Most commonly superficial lobe
Inferior (lower pole) or “tail” of parotid gland
Deep lobe less frequently
Large lesions may compromise airway
Minor salivary glands 2nd most common site
Palate
Most common minor salivary gland site
Involves junction of hard and soft palate
Unilateral, fixed mass (no soft tissue to allow mobility)
Buccal mucosa
Upper lip
Rarely affects lower lip and tongue
Uncommon in submandibular and sublingual glands
Can affect larynx, nasal cavity, ear, orbit, upper aerodigestive tract, gastrointestinal tract
Rarely, may develop within ectopic salivary gland tissue
Presentation
Usually, painless, slow-growing mass
Single, smooth, mobile, firm nodule
Rarely, a 2nd tumor is found
Metachronous vs. synchronous
May be identified concurrently with Warthin tumor
Mucosal ulceration is uncommon
Paresthesia due to nerve compression is rare finding
If pain is present, tumor is more likely to be infarcted Natural History
Slow growing
Asymptomatic
May reach enormous size if neglected
Uncommon malignant transformation
Up to 7% of cases
Treatment
Options, risks, complications
Surgical complications
Frey syndrome (gustatory sweating)
Decreased muscle control of face (if facial nerve is sacrificed)
Capsule disruption may result in “seeding” of tumor (increases likelihood of recurrence)
Enucleation only results in high recurrence rate (up to 50%)
Surgical approaches
Parotid gland
Superficial parotidectomy
Extracapsular dissection (include rim of uninvolved tissue)
Facial nerve preservation when possible
Minor glands
Conservative, complete surgical excision
Submandibular gland
Complete excision
Prognosis
Excellent long-term prognosis, although limited by recurrence and malignant transformation
Overall recurrence rate: Up to 2.5%, most developing within 10 years
Parotid gland tumors have recurrence rate as high as 8%
Recurrences tend to be multinodular or multifocal
Submandibular and minor salivary gland tumors rarely recur
Malignant transformation in up to 7% of cases, with the following risk factors
Long history of untreated tumor
Multiple recurrences
Age of patient (usually > 40 years)
Male gender
Tumors > 2 cm in greatest dimension
Deep lobe tumors
More common in parotid gland
IMAGE FINDINGS
General Features
Imaging provides information about exact anatomic site, extent of disease, and possible invasion or nodal metastases
Ultrasound or CT are complimentary and allow for image-guided fine needle aspiration
Excellent resolution and tissue characterization without radiation hazard, especially for superficial lobe lesions
MR or CT is mandatory to evaluate tumor extent and exclude local invasion
Unilateral mass, which shows post-contrast enhancement, has high T2 signal, and does not invade surrounding tissue planes, is most likely PA
MR spectroscopy may separate Warthin from PA, although not yet well accepted
Ultrasonography is especially valuable in children, since most tumors are benign and many are cystic or vascular (color Doppler for latter)
High-resolution sonography has nearly 100% sensitivity in detecting intraparotid tumors
Precisely outlines tumor borders
Can detect multiple or bilateral lesions
Sialography delineates ductal system but is limited in tumor assessment
MACROSCOPIC FEATURES
General Features
Irregular mass
Fibrous capsule
Parotid gland
Variably thick incomplete capsule but rarely unencapsulated
Minor glands
Poorly developed to absent
Cut surface homogeneous, white to white-tan
Recurrent tumors are generally multinodular
Hemorrhage
Secondary to FNA or previous surgical procedures
Infarction
Secondary to FNA or previous surgical procedures
Size
Majority between 2-5 cm
Rarely, may be enormous
MICROSCOPIC PATHOLOGY
Histologic Features
Innumerable architectural patterns
Solid
Tubular or trabecular
Cystic
Epithelial tissue shows variable morphology
Spindle
Clear
Squamous
Basaloid
Plasmacytoid
Mesenchymal-like tissue
Myxoid stroma
Myxochondroid
Hyaline stroma
Rarely lipomatous
Bone
Duct structures
Lined by cuboidal epithelium
Lined by columnar epithelium
Rarely, crystals are present
Collagenous crystalloids: Eosinophilic needle shapes arranged radially
Tyrosine-rich crystalloids: Eosinophilic bunted shapes arranged tubularly
Crystalloids resembling oxalate crystals
Occasionally squamous metaplasia is identified
Rarely necrosis
Rarely sebaceous cells
ANCILLARY TESTS
Cytology
Findings are variable
Cellular smears with epithelial and mesenchymal cells and background
Clusters or cohesive groups of epithelial cells
Branching trabeculae of cells that drop off into stroma
Plasmacytoid or spindle cells
Bipolar myoepithelial cells with eccentric round nuclei
Spindled cells tend to embed within stroma
Round, ovoid to fusiform nuclei
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree