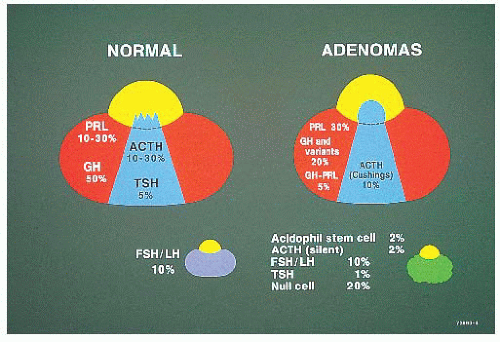
TABLE 12.1 Most Common Tumors and Tumorlike Lesions of the Pituitary Gland and Sellar Region | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
and export. Identification of normal and neoplastic pituitary cell types requires attention to such features as cell shape; the content and disposition of organelles; the presence or absence and arrangement of filaments; and of course, the number, size, electron density, and shape of secretory granules (Table 12.4). The mechanisms of hormone secretion vary from transmembrane diffusion to the actual expulsion of secretory granules, a characteristic of PRL cells (7).
TABLE 12.2 Normal Anterior Pituitary Gland: Cellular and Hormonal Features | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
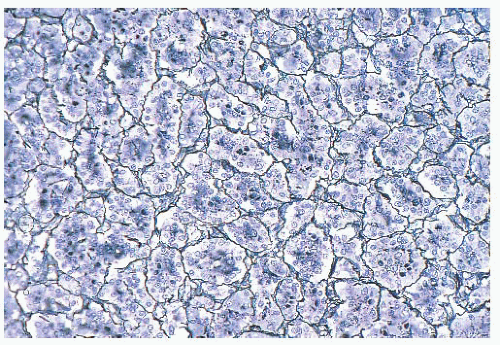
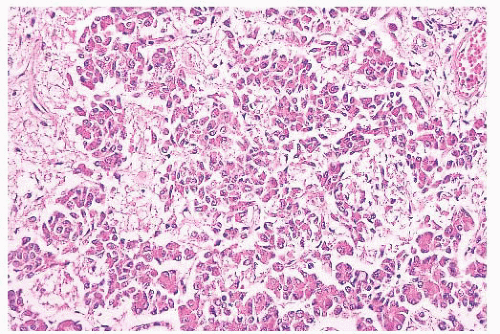
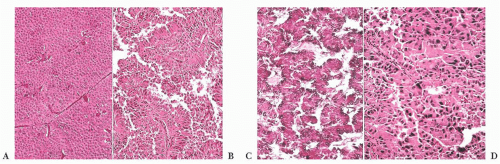
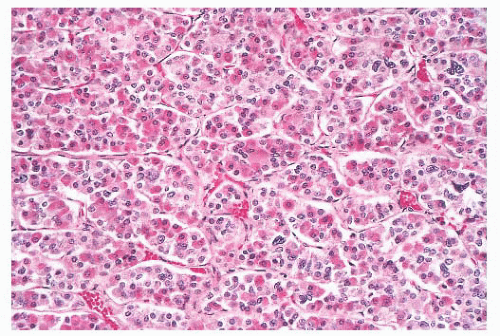 FIGURE 12.2 Normal anterior pituitary. Note the variation in cellular granularity. The staining ranges from acidophilic to chromophobic; several dark-staining basophils are also present. |
TABLE 12.3 Pituitary Adenomas: Clinical and Pathologic Characteristics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 12.4 Pituitary Adenomas: Ultrastructural Features | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
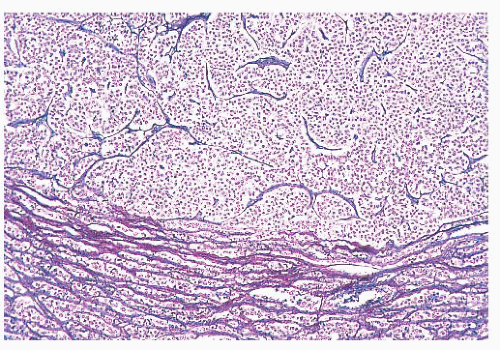
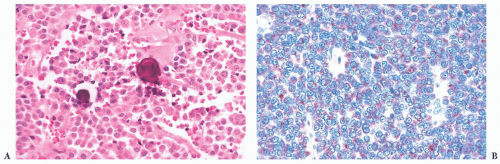
(ACTH-secreting cells). Their accumulation with age, a process termed basophil invasion of the posterior lobe, is of unknown clinical significance. Such cells are believed by some to give rise to so-called silent corticotroph cell adenomas (5,11).
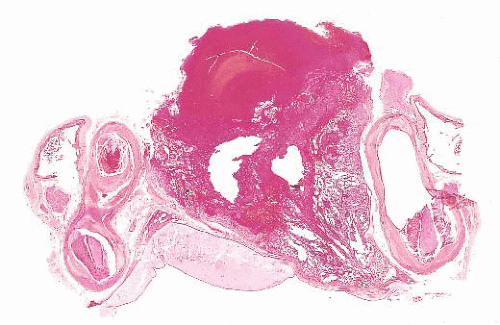
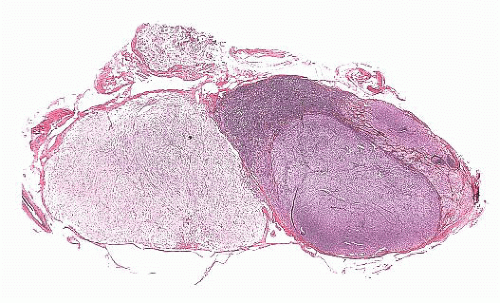
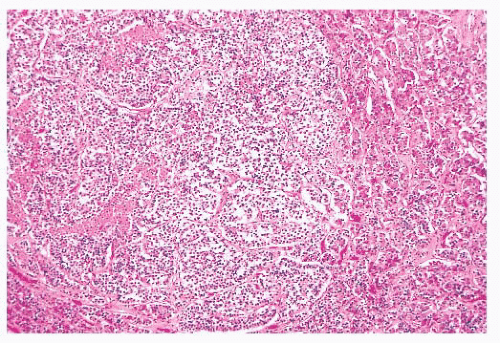 FIGURE 12.8 Microadenoma. Relative circumscription and early compression of surrounding parenchyma are seen. The acinar architecture is effaced. |
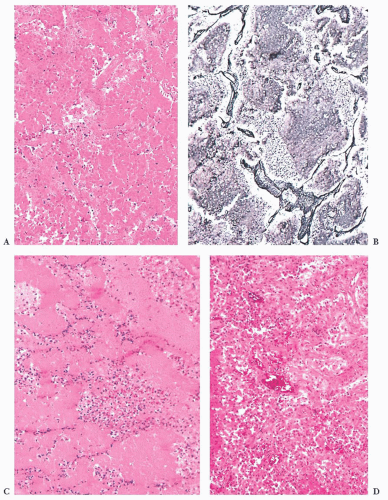
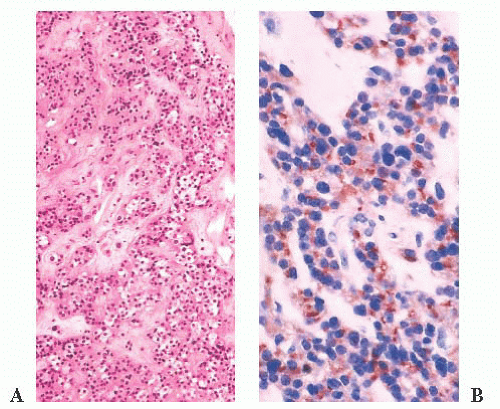
prone. The specimens usually consist of blood and necrotic tumor. Identification of the underlying tumor is aided by reticulin stains, which highlight the abnormal stromal pattern of the adenoma (Fig. 12.11).
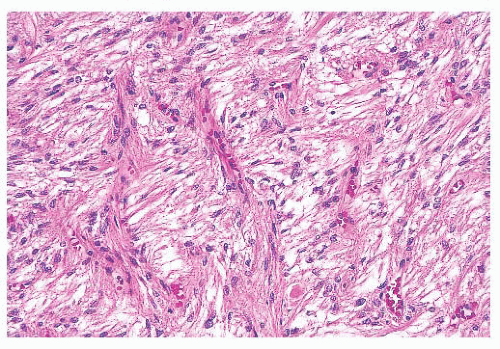
cells. Although the majority of prolactinomas are medically treated by dopamine agonists, a significant number of the patients undergo surgical resection due to several clinical issues (17,18).
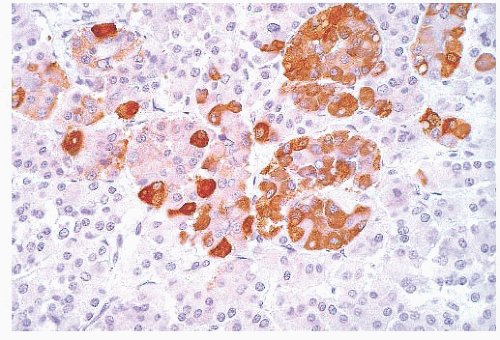
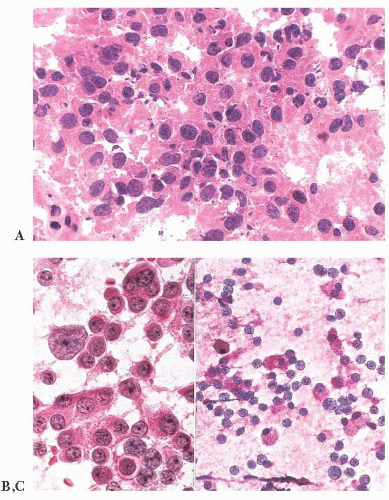
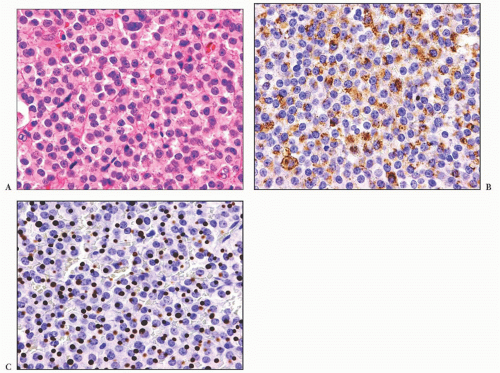
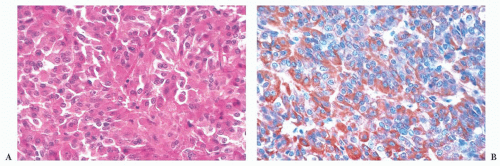
does the rare finding of spherical amyloid bodies (Fig. 12.17) (50). Immunoreactivity for PRL is typically strong but is paranuclear in location (Fig. 12.16). The ultrastructural features of PRL cell adenoma are distinctive (Table 12.4) and include abundant rough endoplasmic reticulum, as well as “misplaced exocytosis” or granule extrusion between neoplastic cells (37,38).
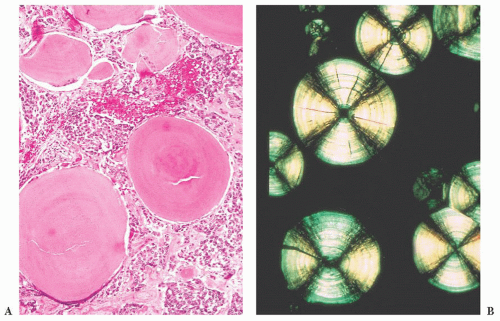 FIGURE 12.17 Prolactinoma with amyloid deposition. Such spherical bodies are virtually diagnostic of a prolactin cell adenoma (B, polarization). |
(IGF-1) produced by the liver and more reliably elevated than GH levels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree