Chapter 21 Phenols
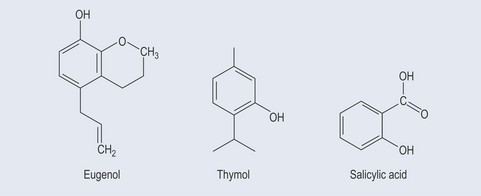
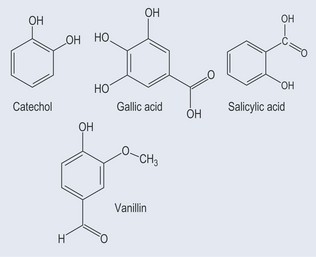
Phenols constitute probably the largest group of plant secondary metabolites, varying in size from a simple structure with an aromatic ring to complex ones such as lignins. Although many of the essential oils are terpenes, some are phenolic compounds, for example thymol from Thymus spp. (thyme) (Figure 21.1). Many simple phenols are responsible for taste, for example eugenol in cloves. They are called the phenylpropanoids because they originate from phenylalanine (see Figure 11.1, p. 82) and they have a six-carbon (C6) and three-carbon (C3) structure (Figure 21.2). They comprise several groups:
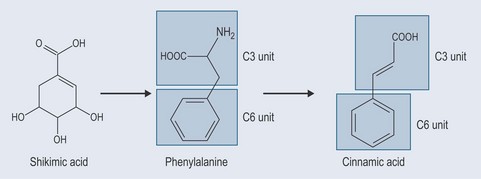
Figure 21.2 Showing the development of simple phenylpropenes from shikimic acid and demonstrating the C3 and C6 units.
Many of these are attached to sugars that have to be cut or cleaved before activity (see Chapter 24 ‘Glycosides’, p. 181).
Shikimic Acid
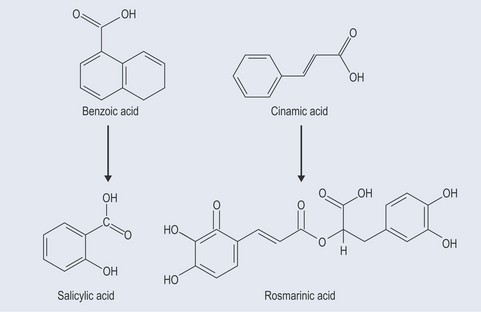
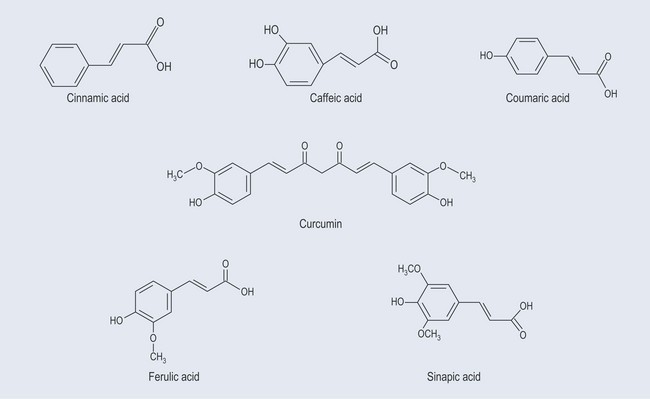
The starting point for the production of phenylpropanoids is shikimic acid (Figure 21.2). Shikimic acid (or shikimate) is the precursor for:
Abridged Phenylpropanoids
These possess no side chain or a side chain with one carbon atom (Figure 21.3).
Simple Phenols
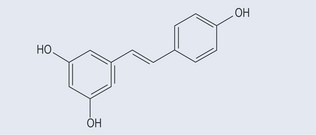
Stilbenes
These are found in the heartwood of plants. They are thought to be responsible for ‘French paradox’ whereby deaths from heart disease in France are lower than in other countries. Resveratrol (Figure 21.6), a component of red wine, acts not only as an antioxidant and an anti-inflammatory but also as an antitumour agent. It is found in blueberries, bilberries, cranberries, and in the skins and seeds of grapes.
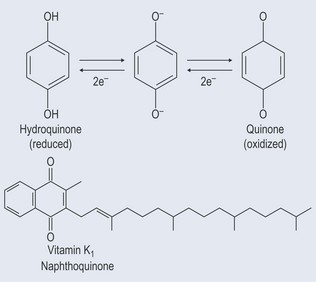
Quinones
Derived from the oxidation of phenols, quinones are closely involved in photosynthesis because they have the ability to gain and lose electrons, which enables the conversion of light energy in a form of energy a plant can use (see Chapter 2 ‘Atoms’, p. 10). They are able to carry electrons between the components that are involved in light reactions (Figure 21.7). Quinones are very lipid soluble.
Naphthoquinones
Naphthoquinones are not widespread but are found in:
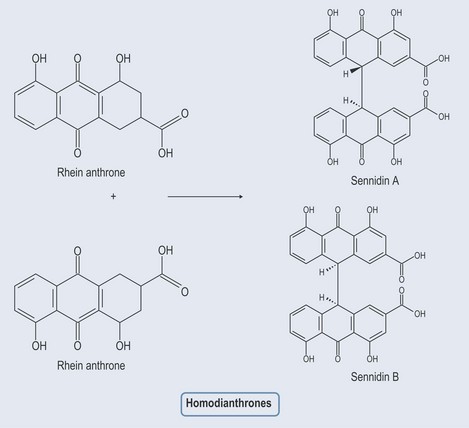
Anthraquinones
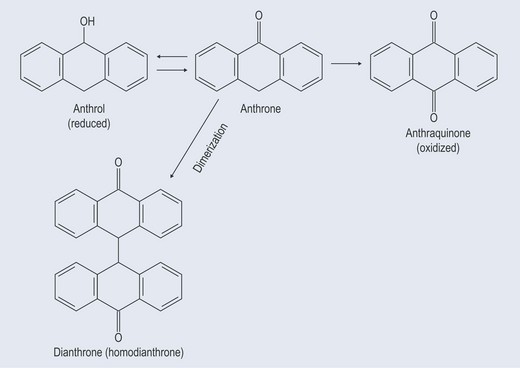
Figure 21.8 Diagram showing the relationship between anthraquinones, anthrones, dianthols and dianthrones.
• Action of Anthraquinones
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree