Fig. 15.1
Improvement in migraine during pregnancy: outcomes of retrospective and prospective studies
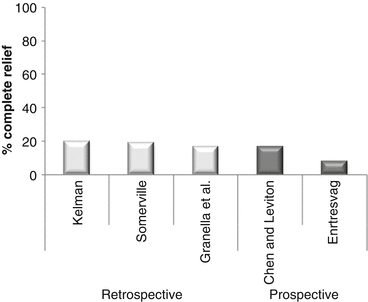
Fig. 15.2
Complete relief of migraine during pregnancy: outcomes of retrospective and prospective studies
Table 15.1
Effect of pregnancy on women with prior history of menstrual vs nonmenstrual headaches
Study | Sample size | Improvement | |
|---|---|---|---|
Hx menstrual headache (%) | Hx nonmenstrual headaches (%) | ||
Melhado et al. [11] | 933 | 78 | 55 |
MacGregor et al. [7] | 30 | 71 | 31 |
Lance and Anthony [6] | 120 | 64 | 48 |
Granella et al. [4] | 571 | 22.7 | 16.8 |
Migraine can be troublesome in early pregnancy but usually improves by the end of the first trimester; if it is still troublesome early in the second trimester migraine, it is likely to persist throughout pregnancy [14]. Aura can occur for the first time during pregnancy and requires specific assessment if the symptoms are atypical [15, 16]. A careful history is imperative to differentiate migraine aura from other transient neurological disorders [17]. The differential diagnosis of atypical aura or persistent headache in pregnancy is thrombocytopenia, cerebral venous sinus thrombosis, or imminent eclampsia.
Postpartum is also a time of increased risk of migraine, typically occurring a couple of days following delivery [14, 18, 19]. Breastfeeding should be encouraged, where possible, as it sustains the benefits of pregnancy on migraine until menstruation returns [14].
15.3 Effect of Migraine and Lactation on Pregnancy
Migraine itself has no significant adverse effects on the outcome of pregnancy [20, 21]. However, large case-control studies confirm a 1.4-fold increased risk of preeclampsia in pregnancy women with migraine [21, 22].
Migraine is also a recognized risk factor for pregnancy-related ischemic stroke (OR range 7.9–30.7 versus nonmigraineurs) [23].
Although it has not been established if the type of migraine is important with respect to risk of preeclampsia and stroke during pregnancy, there is an increasing body of evidence to support that the risk is associated with migraine with aura and not migraine without aura [24].
15.4 Investigations
Unnecessary investigations can be avoided by taking a careful history. Pregnancy should not affect the decision to investigate; the indications for investigation of the pregnant women with headache are the same as for a nonpregnant woman. MRI is preferred to X-ray exposure and is considered to be safe during pregnancy [25]. Gadolinium can be used if contrast imaging is indicated provided that the lowest risk and lowest dose required of gadolinium is used; high-risk gadolinium-based contrast agents and iodinated contrast media should be avoided [26, 27].
15.5 Management
15.5.1 Nonpharmacological
Where possible, trigger identification and management, small and frequent meals, keeping hydrated, taking regular exercise, and a regular sleep schedule can reduce the frequency of attacks and minimize the need for medication.
When an attack starts, a sweet fizzy drink, resting in a quiet and darkened room, cold and/or hot compresses, and gentle massage can help to ease symptoms.
15.5.2 Pharmacological
Recommendations for the safety of drugs during pregnancy are based on the US Food and Drug Administration (FDA) pregnancy labeling, which has five categories: A, B, C, D, and X (Table 15.2). Safety of drugs during lactation is based on data from the National Library of Medicine Drugs and Lactation Database (LactMed). Evidence of efficacy in migraine management is based on recommendations from the American Academy of Neurology, American Headache Society, and the European Federation of Neurological Sciences [28–30].
Table 15.2
FDA pregnancy categories
Category A Adequate and well-controlled studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy (and there is no evidence of risk in later trimesters) |
Category B Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women |
Category C Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks |
Category D There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks |
Category X Studies in animals or humans have demonstrated fetal abnormalities and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use of the drug in pregnant women clearly outweigh potential benefits |
15.6 Acute Treatment
Most drugs used for acute treatment of migraine can be safely continued during pregnancy and lactation, with the exception of ergots (Tables 15.3 and 15.4). However, as drugs are not licensed for use in pregnancy and lactation they should only be considered if nondrug treatments have failed and the potential benefits to the individual woman outweigh the potential risks to the fetus.
Table 15.3
Drugs used for acute treatment during pregnancy
Level of evidence of efficacya | ||
|---|---|---|
FDA category B: No evidence of harm | ||
Aspirin plus paracetamol plus caffeine | 1st and 2nd trimesters only. Contraindicated in 3rd trimester | A |
Diclofenac | 1st and 2nd trimesters only. Contraindicated in 3rd trimester | A |
Ibuprofen | First line NSAID. 1st and 2nd trimesters only Contraindicated in 3rd trimester | A |
Naproxen | 1st and 2nd trimesters only Contraindicated in 3rd trimester | A |
Tolfenamic acid | More commonly used NSAIDs preferred | B |
Metoclopramide | B | |
Paracetamol | First-line analgesic of choice throughout pregnancy | C |
FDA category C: Benefits outweigh risks | ||
Aspirin | 1st and 2nd trimesters only Contraindicated in 3rd trimester | A |
Almotriptan | A | |
Eletriptan | A | |
Frovatriptan | A | |
Naratriptan | A | |
Rizatriptan | A | |
Sumatriptan | First-line triptan | A |
Zolmitriptan | A | |
Domperidone | B | |
Prochlorperazine | B | |
Prednisolone | U | |
FDA category X: Contraindicated | ||
Dihydroergotamine | A | |
Table 15.4
Drugs used for acute treatment during lactation
Level of evidence of efficacya | ||
|---|---|---|
Minimal risk | ||
Diclofenac | A | |
Eletriptan | A | |
Ibuprofen | First-line NSAID | A |
Sumatriptan | First-line triptan. Consider for severe unresponsive attacks. | A |
Domperidone | Increases milk production | B |
Prochlorperazine | B | |
Tolfenamic Acid | More commonly used NSAIDs preferred | B |
Paracetamol | First-line analgesic of choice | C |
Magnesium sulfate | U | |
Prednisolone | Doses up to 50 mg daily unlikely to cause any adverse effects | U |
Benefits likely to outweigh risks | ||
Naproxen | Drugs with short half-life preferred | A |
Metoclopramide | Increases milk production Avoid use in women with a history of major depression | B |
Risks likely to outweigh benefits | ||
Aspirin | A | |
Aspirin plus paracetamol plus caffeine | A | |
Insufficient data | ||
Almotriptan | A | |
Frovatriptan | Long half-life; drugs with short half-life preferred | A |
Naratriptan | A | |
Rizatriptan < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||