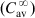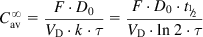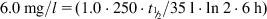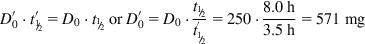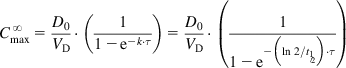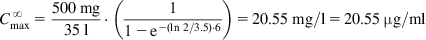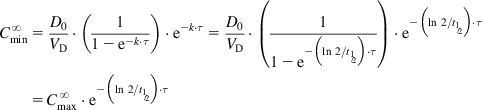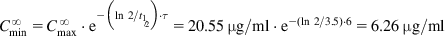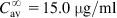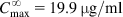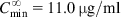Pharmacologic Response and Drug Dosage Adjustments
Drug dosage regimens are generally based on average pharmacokinetic parameters determined in normal patient populations. However, these parameters may vary with patients’ gender, age, weight, and disease state. Other factors such as nutrition and drug co-administration, as well as genetic variability, will also affect the pharmacokinetic parameters. This chapter explains how drug plasma concentrations are maintained within the therapeutic window through clinical observations and adjustment of dosage regiments. It also explains the relationship between drug plasma concentration and the pharmacologic response.
Keywords
Agonist; antagonist; dosage adjustment; dialysis; pharmacodynamics; therapeutic drug monitoring
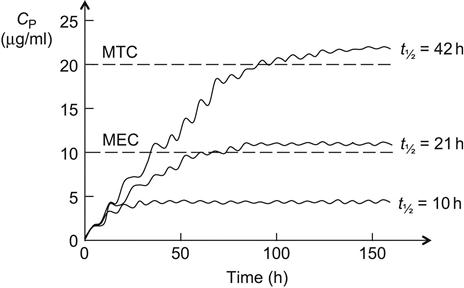
In general, drugs are administered on the basis of their average pharmacokinetic parameters in normal patient populations. Frequently, this is acceptable, achieves the desired therapeutic effect, and does not cause any adverse effects. Many drugs such as most over-the-counter (OTC) drugs and some antibiotics (e.g., penicillins and tetracyclines) have a wide therapeutic window (therapeutic index [TI] or therapeutic range; see Sections 1.1 and 2.7) and are easily maintained within the window, whereas others such as digoxin, aminoglycosides, antiarrhythmic drugs, and anticonvulsants (e.g., phenytoin) have a narrow therapeutic window and are more difficult to administer. In some cases, a strict individualization of the drug dose may be required. Figure 5.1 shows calculated phenytoin CP–time curves in three different individuals, with phenytoin metabolized at an average rate (t½=21 h), fast (t½=10 h), and slowly (t½=42 h), when one 100 mg tablet is given three times a day (D0=100 mg, t=8 h). After the drug has been administered for about 3 days, the CP reaches the minimum effective concentration (MEC) in the patient with t½=21 h, and the therapeutic effect is observed. The CP never goes above minimum toxic concentration (MTC), and thus toxic side effects are never observed in the patient. In the fast-metabolizing patient, CP never goes above the MEC and consequently, the therapeutic effect is never observed in this patient. The dosage (D0) has to be increased (e.g., D0=250 mg, τ=8) to elevate CP above the MEC. In the slow-metabolizing patient, the therapeutic effect is observed after the drug has been administered for about 2 days, but after 4 days, toxic side effects start to be observed. Thus, the dose has to be lowered in the slow metabolizer (e.g., D0=50 mg, τ=8).
5.1 Therapeutic Drug Monitoring
Pharmacokinetic parameters of drugs such as rate constants and volumes of distributions may vary with patient’s age, gender, weight, clinical status, nutritional status, genetic variability (slow or fast metabolism), and co-administration of two or more drugs (drug interactions). Therapeutic drug monitoring (TDM) is a branch of clinical pharmacokinetics that involves determination of plasma drug concentrations and subsequent change of the drug dosage to maintain plasma concentrations within a targeted therapeutic window or, in other words, to tailoring a dose regimen to an individual patient. TDM may be needed when during drug therapy, the patient does not show a proper therapeutic response or displays toxic drug reactions that can be related to low or high drug plasma concentrations (CP). TDM is also used to monitor for a patient’s compliance, that is, to check if the patient follows the prescribed drug regimen. In the beginning, patients receive standard dosage regimens based on information obtained from, for example, clinical handbooks and the drug manufacturer. If the patient displays inadequate therapeutic response or toxic symptoms, blood samples are collected and the drug plasma concentration determined. This is followed by pharmacokinetic evaluation of the data and dosage adjustment.
5.2 Dosage Adjustments
Kidneys and liver are the most important organs for drug elimination from the body. Any changes in their function will affect the drug t½, as well as other pharmacokinetic parameters, and dosage adjustments will be required to maintain CP within the therapeutic concentration range. Decreased kidney function (i.e., renal failure) can be due to, for example, infection, diabetes, or nephrotoxic drugs. It is mainly observed as a decreased glomerular filtration rate (GFR, see Section 2.6.2) or, in other words, decreased or absence of urine production. Dosage adjustments are also performed during dialysis (hemodialysis) when the blood of patients with acute kidney failure is treated in a dialysis machine (i.e., artificial kidney). Decreased liver function (i.e., hepatic failure) can be caused by, for example, excessive alcohol consumption, drug overdose (e.g., acetaminophen [paracetamol] overdose), and viral infection (e.g., hepatitis). Furthermore, both kidney and liver functions are affected by aging. The GFR declines with increasing age as does the metabolic rate; thus, older people are, in general, given smaller drug doses compared with young adults or middle-aged persons. Likewise, infants, especially newborns, do not have fully developed enzyme systems and generally require relatively small drug doses. Furthermore, newborns show only 30–40% renal activity, according to body weight, in comparison with adults.
The equations used to adjust drug dosage are based on keeping the same 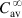 in normal and affected persons, as described by Eq. (2.135), assuming that drug bioavailability is not affected (i.e., F=F′):
in normal and affected persons, as described by Eq. (2.135), assuming that drug bioavailability is not affected (i.e., F=F′):
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree