Chapter 27 Over the past 30 years there has been a progression from large-incision intracapsular surgery to less invasive small-incision extracapsular surgery. Similarly, glaucoma surgery has progressed from full-thickness fistulizing procedures (Scheie and posterior lip sclerectomy) to guarded fistulizing procedures (trabeculectomy), and then to trabeculectomy with antimetabolite usage, and currently deep sclerectomy and viscocanalostomy (nonpenetrating trabecular surgery). This chapter discusses the complications of phacotrabeculectomy and the new phacoglaucoma procedures with emphasis on the avoidance, recognition, and intervention of potential complications. There are certain preoperative conditions that pre-dispose to complications in both trabeculectomy and nonpenetrating trabecular surgery (NPTS). Medications that promote bleeding are the nemesis of the glaucoma surgeon (Table 27–1). Specifically, these include Coumadin (warfarin sodium), aspirin and other oral nonsteroidal antiinflammatory drugs (NSAIDs), vitamin E in high doses,1,2 platelet aggregation inhibitors other than NSAIDs including Ticlid (ticlopidine hydrochloride) and Plavix (clopidogrel bisulfate), pilocarpine, and other miotics. The mechanism of action of Coumadin is inhibition of blood clotting by interference with the hepatic synthesis of the vitamin K–dependent clotting factors (II, VII, IX, and X).3 Aspirin irreversibly inhibits platelet aggregation for the 8- to 10-day lifetime of the affected platelets.4 This causes a prolonged bleeding time that in the clinical experience of this author is more difficult to control than bleeding caused by Coumadin at a therapeutic level. Oral NSAIDs other than aspirin, such as ibuprofen, naproxen, and ketoprofen, reversibly inhibit platelet aggregation and therefore platelet function returns when most of the drug has been eliminated from the patient’s body.4 A new class of NSAIDs, the cyclooxygenase (COX-2) inhibitors, does not affect platelet function because it mainly inhibits only one isoform of the enzyme COX-2.5 The older NSAIDs inhibit both COX-1, which is found in large amounts in platelets, and COX-2, which is minimally present in platelets, but exists in other tissues.5 Vitamin E may interfere with vitamin K metabolism and platelet function and therefore increase the bleeding tendency.1,2 There is currently a trend toward taking antioxidant vitamins. Therefore, the ophthalmologist should inquire about vitamin E usage during the preoperative planning visit. Ticlid and Plavix are both platelet aggregation inhibitors that are used for secondary prevention of myocardial infarction, stroke, and other vascular events.6,7 Unlike aspirin that inhibits cyclooxygenase and formation of prothrombotic thromboxane in platelets and antithrombotic prostacyclin in vessel walls, Ticlid and Plavix inhibit platelet aggregation by inhibiting the adenosine diphosphate (ADP) pathway for platelet activation.6,7 Both Ticlid and Plavix prolong the bleeding time similarly to aspirin.6,7
PHACOTRABECULECTOMY AND
OTHER GLAUCOMA PROCEDURES
PREOPERATIVE CONDITIONS THAT PREDISPOSE TO COMPLICATIONS
Medication | Accepted Time to Discontinue |
Coumadin (warfarin sodium) | 3 to 5 days |
Aspirin | 10 to 14 days |
Oral nonsteroidal antiinflammatory drugs (NSAIDs) | 3 to 5 days |
10 days to 2 weeks | |
Ticlid (ticlopidine hydrochloride) | 20 days to 2 weeks |
Plavix (clopidogrel bisulfate) | 10 days to 2 weeks |
Pilocarpine (and other miotics) | 3 weeks |
Pilocarpine and other miotics (both direct and indirect acting) promote bleeding by causing vasodilatation in the ocular tissues and breakdown of the blood–aqueous barrier. This causes increased fibrin to be released into the anterior chamber and the ocular tissues increasing the likelihood of failure of the trabeculectomy or the NPTS.
Bleeding is undesirable in glaucoma surgery because it promotes inflammation and scar tissue formation. This can cause the glaucoma procedure to fail. To minimize the risk of bleeding, aspirin should be stopped at least 10 to 14 days prior to surgery.2
Older NSAIDs other than aspirin should be stopped at least 3 to 5 days prior to surgery to allow the platelets to recover. The new NSAIDs, which are COX-2 inhibitors Vioxx (rofecoxib) and Celebrex (celecoxib), do not need to be stopped prior to surgery because they do not inhibit platelet function at therapeutic doses.5
It is reasonable to stop Coumadin 3 to 5 days prior to surgery if this is acceptable to the patient’s primary care physician. If this is not acceptable, or if the patient’s prothrombin time (PT) does not normalize by the day of surgery when off Coumadin, then the patient may be given fresh frozen plasma (FFP) several hours prior to surgery. From a practical standpoint, FFP rarely has to be given because most of the time glaucoma surgery is elective rather than emergent and therefore can be postponed until the PT normalizes.
It also seems prudent to discontinue vitamin E for 10 days to 2 weeks prior to surgery to reverse the vitamin’s effect on platelets,2 especially if a patient is taking 400 to 800 IU a day, which is seven to 14 times as much as the recommended daily allowance for vitamin E.
Ticlid and Plavix should be stopped 10 days to 2 weeks prior to surgery because it takes at least 7 to 10 days and sometimes up to 2 weeks for platelet function to return to normal after stopping these drugs.6,7
It is desirable to discontinue miotics for at least 3 weeks prior to trabeculectomy or NPTS and longer if possible.8 Usually, by starting Diamox 500 mg sequels po b.i.d., it is possible to discontinue miotics for the 3-week period without having an unacceptable rise in intraocular pressure. This may not be possible based on the severity of a patient’s glaucoma and the clinical response to the discontinuance of the miotic. Frequently, a different topical medication can be substituted for a miotic to allow the discontinuance of the latter prior to surgery.
MIOTIC AND OTHER TOPICAL EYEDROP USAGE
Not only do miotics cause an increase in bleeding and inflammation at the time of glaucoma surgery, but they and other antiglacuoma medications cause morphologic changes to occur in the conjunctiva, Tenon’s fascia, and episclera. These changes, according to clinical studies, have a negative effect on the outcome of filtration surgery.9,10 They include epithelial meta-plasia, a decrease in goblet cells, and an increase in subepithelial fibroblasts and inflammatory cells (macrophages, lymphocytes, and mast cells).10–12 Pilocarpine and epinephrine have been the main offenders but other topical agents may cause conjunctival changes as well. One study revealed that a commercial preparation of 0.5% timolol maleate causes a significant decrease in goblet cells after only 1 month of topical treatment.11 The literature supports the concept that the administration of topical antiglacuoma medication, irrespective of type, for more than 3 years causes significant subclinical inflammation within the conjunctiva and foreshortening of the inferior fornix secondary to conjunctival fibrosis.10,13 It is not known whether it is the actual drug or the preservative, such as benzalkonium chloride, or a combination of both that cause these changes. If a patient is on topical antiglacuoma mediation prior to the performance of a trabeculectomy or NPTS, it would seem reasonable to start topical steroids and topical NSAIDs for 3 to 5 days prior to surgery to attempt to diminish inflammation prior to making the incision. Some glaucoma surgeons advocate the use of oral steroids in the peri-operative period but a clinical trial indicating their effectiveness, or a risk analysis, has not yet been done.
OTHER CONDITIONS THAT PREDISPOSE TO COMPLICATIONS
Patients who have had previous ocular surgery in which the conjunctiva, episclera, and sclera have been violated are at increased risk of complications from trabeculectomy and NPTS. Having knowledge of the patient’s previous surgery helps the surgeon formulate a plan of action. When possible, it is desirable to choose a location on the conjunctiva that has not been scarred by previous surgery. This is sometimes not possible after previous intracapsular, planned extracapsular surgery, or glaucoma surgery, where 10 to 14 mm of conjunctiva has been disturbed. This situation would demand that the surgery be performed in the inferior bulbar location with the attendant increased risk of endophthalmitis that exists when a bleb forms. Because a bleb usually doesn’t form after a viscocanalostomy, the inferior bulbar location is theoretically no riskier than a superior location for this operation. However, it is more difficult to operate in the inferior location from an ergonomic standpoint. If the surgeon is forced to operate in nonvirgin conjunctiva, then preoperative evaluation at the slit lamp should include assessment of the conjunctiva with a cotton-tipped applicator to see what clock hours of conjunctiva are the least adherent to the underlying episclera and sclera. This should be noted so that the proposed surgical site is easy to find at the time of surgery.
Patients who are darkly pigmented or young in age have a greater tendency to scar after trabeculectomy. Recording these traits may influence the surgical plan. For example, the surgeon may choose to order 0.4 mg/mL instead of 0.2 mg/mL of mitomycin-C, as well as perform a more aggressive trabeculectomy by making a larger sclerectomy.
Other factors to consider are additional unique characteristics about the patient’s anatomy that may influence the surgical plan, such as a prominent brow, which may necessitate a temporal approach, or a small palpebral fissure, which may require a lateral canthotomy.
SPECTRUM OF GLAUCOMA PROCEDURES
Before entering into a discussion of complications it is first necessary to review the current spectrum of glaucoma surgery as well as the details of the newer procedures. Prior to 1968 when Cairns14 introduced the trabeculectomy operation, full-thickness filtering surgery was the procedure of choice for medically uncontrolled open angle glaucoma. Full-thickness procedures were to a great extent supplanted by trabeculectomy procedures especially when mitomycin-C usage enabled intraocular pressure (IOP) reduction down to the higher end of the range achieved with full-thickness operations. Even though trabeculectomy is currently considered the standard filtration procedure for glaucoma, it is still fraught with some early postoperative complications including hyphema, surgically induced iritis, and excessive filtration leading to a shallow or flat anterior chamber, suprachoroidal effusions, hypotonous maculopathy, and endophthalmitis.15–17 This has prompted the development of several NPTSs, deep sclerectomy,18–21 and viscocanalostomy,22 which seem to reduce the incidence of most of the early complications experienced with trabeculectomy. Another penetrating glaucoma surgery, trabeculotomy, has been utilized for decades primarily in infantile glaucoma. There has been renewed interest in trabeculotomy in conjunction with phacoemulsification and intraocular lens implantation in adults.23 Trabeculotomy does help to minimize certain early postoperative complications of trabeculectomy including shallowing of the anterior chamber, suprachoroidal effusion, and malignant glaucoma, but other complications may be more frequent, such as postoperative IOP spikes.24,25 According to a study by Tanihara et al,23 trabeculotomy success rates were significantly lower when the initial IOP level was greater than 30 mm Hg, and the procedure seemed to be more effective in controlling IOP in eyes with pseudoexfoliation syndrome than in eyes with primary open angle glaucoma. In contrast, the viscocanalostomy operation works in all types of glaucoma except for neovascular. Stegman et al22 report excellent results (<22 mm in 82.7% of blacks without medications) in patients who present with an initial mean IOP level of 47.4 mm. Stegman et al22,26 performed hundreds of trabeculotomies prior to creating the viscocanalostomy operation. These trabeculotomies were performed by several different techniques. The first technique used by Stegman et al was called a trabeculoviscotomy, in which Schlemm’s canal was injected with Healon to rupture the wall of the canal and the trabecular meshwork.26 A subsequent technique involved cutting the canal and meshwork with a very fine pair of scissors.26 Even though their results were fairly good (61% success—IOP <21 in technique 1 and 76% success in technique 2),26 if the operation failed, the significant damage done to Schlemm’s canal and the collector channels precluded a NPTS from being performed in the 160-degree area of sclera involved in the trabeculotomy. It is for these reasons, as well as the fact that NPTS is less invasive with no entrance into the anterior chamber, that this author chooses not to discuss trabeculotomy and its complications in this chapter.
DEEP SCLERECTOMY PROCEDURE
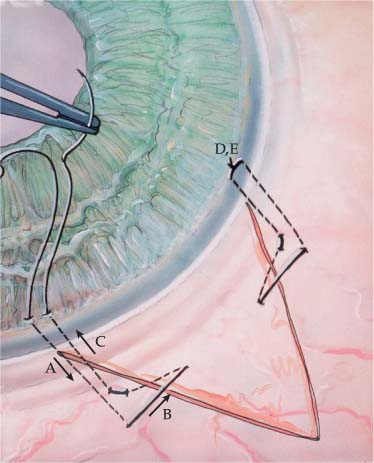
The deep sclerectomy operation proceeds as follows. A limbus- or fornix-based conjunctival flap can be used. The fornix-based flap probably allows for an easier dissection of a superficial scleral flap anteriorly into the clear cornea. This is the second step of the procedure. Cautery can be used to stop episcleral bleeding. The superficial flap should measure at least 5 × 5 mm and can be in any shape, but most surgeons choose a square or semicircle. The flap should be approximately one-third scleral thickness (200 to 250 μm in depth) and should extend anteriorly into the clear cornea 0.5 to 1.00 mm. A second scleral flap measuring approximately two-thirds scleral thickness (500 to 550 μm in depth) is created beneath the superficial scleral flap. It should extend into the cornea 0.5 mm to 1 mm anterior to Schwalbe’s line. The sclera in black patients is approximately 80 μm thicker than in the Caucasian population and this necessitates a thicker deep scleral flap to get into the correct tissue plane.26 This plane is usually evident when the underlying choroid is visible beneath the remaining thin translucent layer of sclera. As the deeper scleral flap dissection proceeds anteriorly, Schlemm’s canal becomes visible as a dark line approximately 1 mm posterior to the limbus. Stegman27 points out that in a highly hyperopic eye, Schlemm’s canal may be located at or near the limbus, as opposed to a highly myopic eye, where it may be located as far posteriorly as 2.5 mm posterior to the limbus. In congenital glaucoma, Schlemm’s canal may be as far as 4 mm posterior to the limbus. Further dissection of this deep flap un-roofs Schlemm’s canal and then reveals Descemet’s membrane.
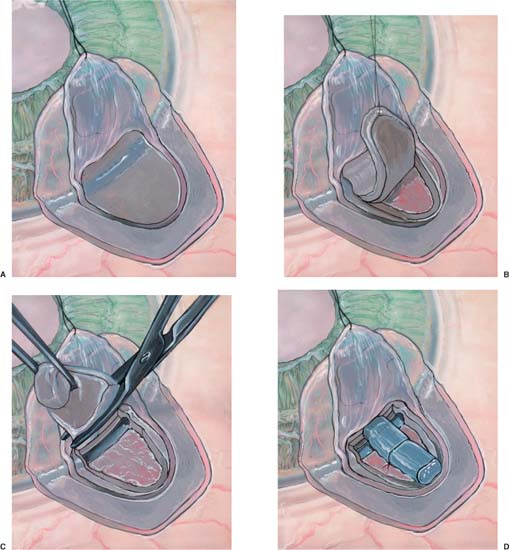
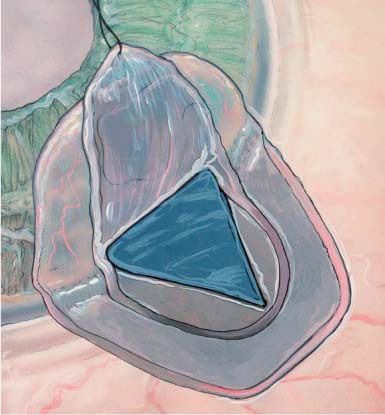
Once the deep scleral flap is advanced into the clear cornea 0.5 to 1.0 mm, it is excised. This deeper scleral flap is usually made triangular in shape whether the superficial flap is square or semicircular. At this time the surgeon may replace the superficial flap without sutures or place the Staar collagen sponge (Aquaflow) in the space created by the deep sclerectomy. The superficial flap is then loosely sutured into position with two 10-0 nylon interrupted sutures.19–21 The sponge is oriented radial to the limbus and sutured into position with one 10-0 nylon suture. Deep sclerectomy with placement of the Staar collagen sponge is depicted in Figure 27–1A–E.19 Another variation in the deep sclerectomy operation is the placement of a reticulated hyaluronic acid implant into the space created by the sclerokeratectomy (Fig. 27–2).28 When the implant is used, the superficial flap is sutured tightly to prevent bleb formation. This approach to deep sclerectomy promotes drainage of aqueous through the two surgically created ostia into Schlemm’s canal, just as in the viscocanalostomy operation. It remains to be seen whether lower IOPs are achieved by injecting sodium hyaluronate into the ostia of Schlemm’s canal or if the hyaluronic implant, which slowly releases sodium hyaluronate for more than 4 months in humans,28 can achieve the same or better result.
VISCOCANALOSTOMY PROCEDURE
The viscocanalostomy procedure has been developed by Stegman, and has been in its current form for the past 5 years.22 The initial part of this procedure is similar to the deep sclerectomy with some exceptions.
First, the superficial scleral flap should optimally be placed between aqueous collector channels. The collector channels are frequently identified by focal pigmentation on the scleral surface.
Second, the aqueous veins, which drain the collector channels, should not be cauterized, because the success of the operation is contingent upon the patency of the veins and the collector channels.22 To control bleeding, Stegman uses a topical drug, POR-8, which is not available in the United States.22 A similar drug, vasopressin, can be applied topically with good results.26
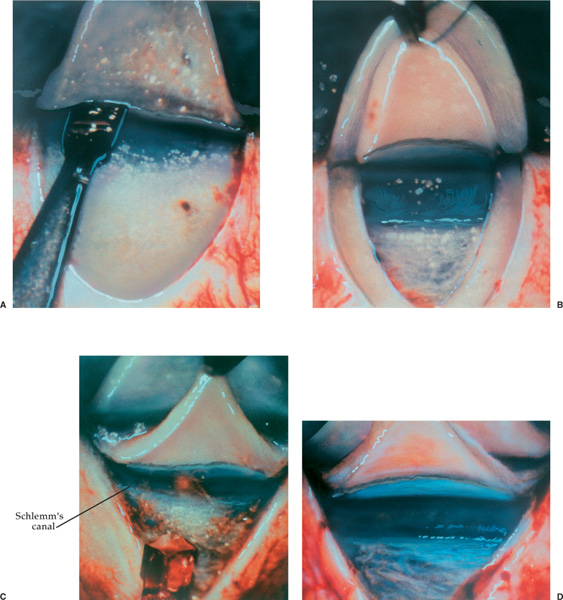
Third, the deep scleral flap dissection should be temporarily halted after Schlemm’s canal has been unroofed. At this time high molecular weight sodium hyaluronate, Healon GV or Healon5, is gently injected into the two ostia of Schlemm’s canal for a distance of 4 to 6 mm on each side.22,26 A special cannula with an outer diameter of 150 μm (Greishaber no. 149.40) facilitates this procedure.22,26 A paracentesis incision should be made, and the anterior and posterior chambers decompressed, prior to the injection of viscoelastic or dissection of the Descemet’s window.22,26 By decompressing the anterior and posterior chambers, the paper-thin Descemet’s window is less likely to be perforated during the anterior dissection of the deep scleral flap.22,26 Decompression prevents iris prolapse into the window if an inadvertent perforation of the window should occur. Finally, if the canal perforates into the anterior chamber because of too forceful an injection of sodium hyaluronate, iris prolapse into the canal is avoided.22,26
The deep scleral flap is then excised, followed by tight suturing of the superficial flap with interrupted 11-0 Mersilene sutures22,26 (Fig. 27–3). Even though both the deep sclerectomy and the viscocanalostomy procedures are nonperforating in nature, the former procedure when performed with the Starr collagen sponge relies on subconjunctival aqueous filtration and bleb formation to be successful in lowering IOP, and the latter procedure does not.
TRABECULECTOMY PROCEDURE
The trabeculectomy operation described by Cairns14 in 1968 has been modified over the years with the addition of antimetabolite usage as well as by the use of externalized releasable sutures.
RELEASABLE SUTURE TECHNIQUES
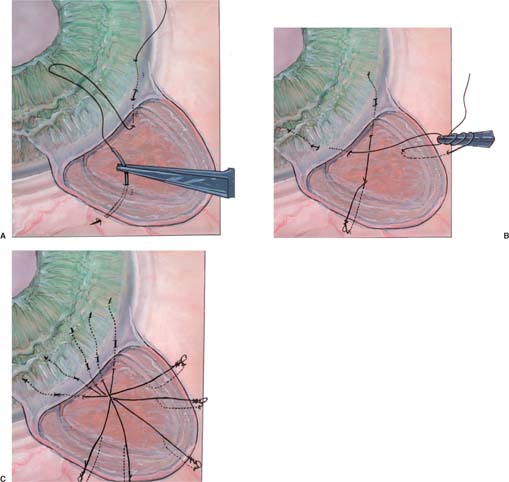
Various releasable suture techniques have been reported by Cohen and Osher,29 Wilson,30 Shin,31 Johnstone and co-workers,32 Hsu and Yarng,33 and Maberley and coworkers.34 These suture techniques may be used with either limbus- or fornix-based conjunctival flaps. The Cohen and Osher technique consists of multiple four throw slipknots of 10-0 nylon, which are used to close the trabeculectomy flap (Fig. 27–4). This suturing technique consists of taking three bites proceeding from the cornea posterior through the scleral flap and emerging from the sclera. The free end of each slipknot is buried within the corneal stroma.
The Wilson releasable technique also involves taking three suture bites with a square knot (3-1-1-1). This is tied on the epithelial surface of the cornea (Fig. 27–5). It is important to cut off the excess suture flush with the knot with a 15-degree blade. This will promote epithelialization of the knot. This is important for patient comfort postoperatively. This suture often produces some astigmatism until it is either released or the nylon relaxes. However, in this author’s experience, it consistently achieves a tighter closure, and is easier to release, than the Cohen and Osher suture. However, the Wilson suture cannot be easily used at the apex of a triangular trabeculectomy flap. The Cohen and Osher releasable suture can. Therefore, it may be helpful to use both suturing techniques in closing trabeculectomy flaps.
Postoperatively these sutures should be released only if the IOP is higher than desired, and then only after some conjunctival and episcleral healing has occurred. Otherwise, hypotony may result. These releasable suture techniques are a valuable adjunct to trabeculectomy surgery and help to decrease the incidence of postoperative hypotony.
COMBINED PHACOEMULSIFICATION AND TRABECULECTOMY
If phacoemulsification with intraocular lens (IOL) implantation is combined with either of the NPTSs or with a trabeculectomy, the cataract incision can be either (1) a separate clear corneal location (a necessity when combined with NPTS because by definition there is no anterior chamber entry), at a site away from the glaucoma surgery, or (2) under the scleral flap of a trabeculectomy (Fig. 27–6).
INTRAOPERATIVE COMPLICATIONS
CONJUNCTIVAL BUTTONHOLE
This complication is most likely to occur if a conjunctival flap is being prepared in tissue that has undergone previous surgery. Subsequent to any surgical procedure, adhesions develop between the conjunctiva and the episclera/sclera, making the dissection demanding. The surgeon should always use blunt scissors and nontoothed forceps when dissecting. When conjunctiva is adherent to underlying tissue, it may be necessary to perform a superficial scleral dissection, removing a thin layer of sclera along with the conjunctiva, to prevent a buttonhole from occurring. The best way to prevent a buttonhole is to choose a surgical site with loosely adherent conjunctiva. If a hole in the conjunctiva does occur, it should be repaired immediately with 8-0 or 9-0 Vicryl interrupted sutures on a blood vessel needle. Purse string sutures work well for this purpose. The needle will minimize the size of the microperforations caused by the needle passage. Incorporating a small amount of Tenon’s fascia into each bite makes the repair more secure. If a large buttonhole occurs, it is reasonable to repair the hole and move to a different site on the sclera, especially if the surgeon is planning on performing a glaucoma procedure that produces a bleb, or if the use of an antimetabolite is planned. If the buttonhole occurs in conjunctiva that is gossamer in nature, sutures sometimes do not hold. If this occurs, abandonment of this surgical site for a new one is advisable, unless the planned procedure is one that does not produce a bleb.
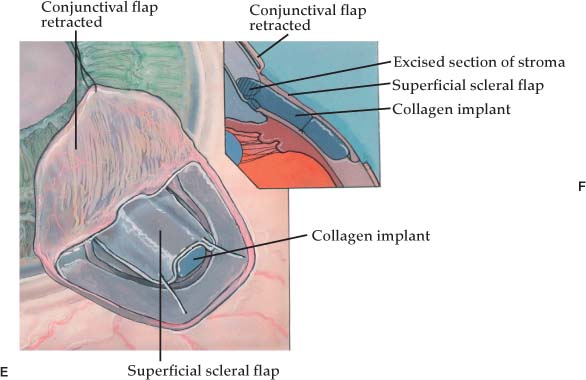
FIGURE 27–1 Deep sclerectomy with Aquaflow collagen implant. (A) Superficial scleral flap, one-third scleral thickness (200 to 250 μm) in depth, 5.0 × 5.0 mm in size extends 1.0 mm into clear cornea. (B) A deep scleral flap, two-thirds scleral thickness (500 to 550 μm) in depth is created within the area of the superficial flap. (C) The deep scleral flap is dissected anteriorally, unroofing Schlemm’s canal and exposing Descemet’s membrane through which aqueous percolates. (D) The Aquaflow collagen implant is sutured radially in the deep scleral bed. (E) Superficial scleral is loosely reapproximated with two 10-0 nylon sutures. (F) (inset) Side view demonstrates relationship of collagen implant to sclera and Schlemm’s canal.
SCLERAL FLAP PROBLEMS
Trabeculectomy
There are a variety of flap complications that can occur in various glaucoma operations. In trabeculectomy surgery the thickness of the scleral flap helps to determine the amount of aqueous egress beneath the flap. A thin flap usually allows more aqueous egress than a thick flap, assuming that other variables (sclerectomy size, number and tension of sutures) are the same. A thin flap is acceptable provided there is adequate tissue for flap closure. This author attempts to create a one-half scleral thickness flap, in trabeculectomy surgery, by creating the outline of the flap with a guarded trifacet diamond blade set at 375 μm. A diamond blade or a metal disk blade is used to dissect the flap. The blade angle is maintained almost parallel to the sclera. If the flap begins to become thin during its creation, redirection of the blade to a deeper plane is easily achievable. This will produce a thicker flap.
If a flap begins to become thick during its posterior dissection, there is a danger of entering the choroid. This is usually evident because the hue of the choroid appears darker as the overlying sclera becomes thinner. Redirection of the diamond blade to a more superficial plane will correct the problem. If the choroid is inadvertently entered, unless there is prolapse of choroid, nothing but redirection of the blade should be done. Only a thin film of sclera is necessary to prevent iris prolapse. If the choroid has a small bulge it can be ignored and the procedure completed in the usual fashion. If however, the bulge is large, the flap must be closed with 10-0 nylon interrupted sutures and a new flap in another location is necessary.
FIGURE 27–2 Reticulated hyaluronic acid implant placed into the space created by the deep sclerectomy space.
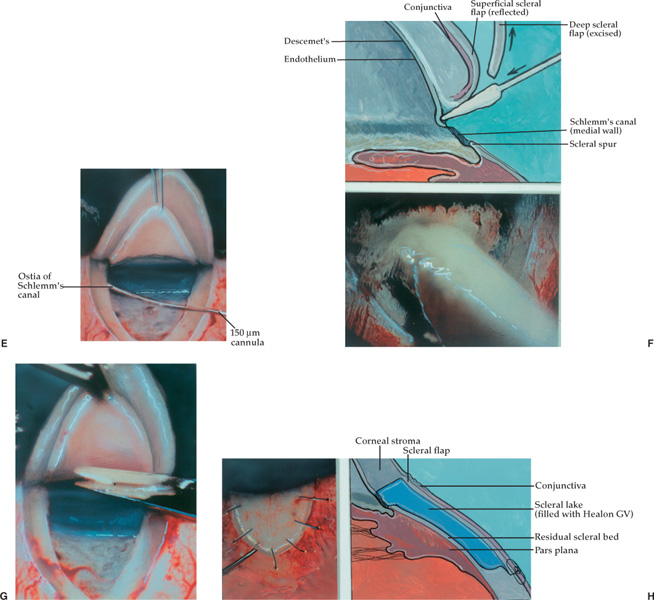
FIGURE 27–3 Viscocanalostomy. (A) A superficial scleral flap (one-third scleral thickness) and parabolic in shape (5.0 × 5.0 mm) is dissected. (B) A deep scleral flap (two-thirds scleral thickness) and parabolic in shape (4.0 × 4.5 mm) is created 0.5 mm inside the borders of the superficial flap. (C) As the deep scleral flap is dissected anteriorly, a dark line becomes visible that is Schlemm’s canal overlying the trabecular mesh-work. (D) Further dissection of the deep scleral flap unroofs Schlemm’s canal. The roof or outer wall of the canal is adherent to the underside of the deep scleral flap and is seen as a slate-gray band. (Adapted from Stegman et al,22 with permission.) (E) A small cannula (150 μm outer diameter) is used to inject Healon GV or Healon5 into the surgically created left ostium of Schlemm’s canal. The cannula is not inserted more than 1/2 to 1 mm. (F) Light pressure is applied with a cellulose sponge to Descemet’s membrane while simultaneously pulling on the deep scleral flap with a 0.12 forceps. This gently separates Descemet’s membrane from the deep scleral flap. (F, top) Diagram of weck cell gently separating Descemet’s from the cornea. (F, bottom) Photo of the same step. (G) The deep scleral flap is excised with a Vannas scissors exercising care not to rupture Descemet’s window. (H, left) Superficial flap is sutured into position securely with 11-0 Mersilene sutures. Healon GV or Healon5 is gently injected under the flap to create the scleral lake. (H, right) Side view showing relationship of superficial scleral flap and deep sclera and Descemet’s window to scleral lake. (Adapted from Stegman et al,22 with permission.)
FIGURE 27–4 (A) Cohen and Osher externalized releasable sutures to close a trabeculectomy flap. (1) First suture bite is a 2- to 3-mm superficial intracorneal bite that is almost parallel to the limbus. (2) Second suture bite enters the cornea 1/2-mm away from the exit site of the first suture bite. The suture passes into the cornea, into the corneoscleral limbus and out through the trabeculectomy flap (close to the base of the flap). (3) Third suture bite passes through the edge of the trabeculectomy flap into the adjacent sclera. (B) The suture is then tied tightly with a four-throw slipknot. The loop is made smaller by pulling on the corneal aspect of the suture. This will facilitate later release. (C) All sutures are in position. The excess suture is excised at the entrance of the intracorneal bite to ensure epithelialization of the suture.
IRIS PROLAPSE
If the scleral flap becomes too thick as dissection proceeds anteriorly near the limbus, then premature entry into the ciliary body, scleral spur, or trabecular meshwork can occur. This isn’t a big problem, especially if the cataract surgery has already been performed at a separate site. However, if the cataract is going to be removed from beneath the scleral flap, iris prolapse is a likely event. The performance of a peripheral iridectomy, prior to phaco, will prevent recurrent iris prolapse, enabling the cataract surgery to proceed. If this is inadequate, a Sheets’ glide may be placed such that it will hold the iris in position and phaco can proceed over it. It may be helpful to hold the glide in place with a temporary scleral suture. If the eye is hyperopic, with a crowded anterior chamber, iris prolapse around the phaco tip can still occur. Under these conditions, a 10-0 nylon suture placed radially in the scleral bed to decrease the size of the surgical ostium can help to keep the iris in the eye.
FIGURE 27–5 Wilson externalized releasable suture. (A) Bite 1 enters the cornea 1 mm from the conjunctival reflection and passes into the corneoscleral limbus and out the sclera adjacent to the trabeculectomy flap. (B) Bite 2 enters the sclera of the trabeculectomy flap near the edge of the flap and into the sclera adjacent to the trabeculectomy flap. (C) Bite 3 enters the sclera adjacent to the trabeculectomy flap, passes through the corneoscleral limbus, and exits the cornea 1 mm posterior to the conjunctival reflection and 1 to 2 mm nasal to the entrance site for bite 1. (D) Suture is tied with 3-1-1-1 throw pattern. (E) Excess suture is cut on the knot with a 15-degree blade to ensure epithelialization.
The best way to prevent iris prolapse is to not get too deep when creating the trabeculectomy flap and to enter the anterior chamber 1 mm anterior to the anterior edge of the scleral flap. This puts the entrance into the anterior chamber approximately 1 to 1.5 mm anterior to the Schwalbe’s line (Fig. 27–6).
Trabeculectomy-Sclerectomy Variables
The size and location of the posterior lip sclerectomy, relative to the size of the overlying scleral flap, is a major determinate of the amount of aqueous egress beneath the flap. If the sclerectomy is large, extending the entire width or length of the overlying flap, then aqueous egress will be greater than if the sclerectomy is smaller. If the sclerectomy is small relative to the overlying scleral flap, then less aqueous egress is usually present and fewer sutures may be required. If the sclerectomy is large, there is a greater chance for postoperative hypotony. If it is small, there is a greater chance that postoperative IOP will be higher initially and possibly more difficult to lower. This is due to significant overlap of sclera between flap and bed. When the sclerectomy is small, and centered, suture release doesn’t necessarily expose the sclerotomy space. Wilson35 has promoted a technique that minimizes the risk of postoperative hypotony, but maximizes the chance of being able to lower the IOP if necessary in the postoperative period. This technique involves making the sclerotomy as wide as the overlying flap on one side rather than both sides. Both sides of a triangular flap are sutured tightly, with interrupted or removable sutures, to minimize aqueous egress and prevent early postoperative hypotony. If IOP needs to be lowered in the postoperative period, laser suture lysis or removal of externalized releasable sutures on the side of the flap adjacent to the sclerectomy site will expose the underlying sclerotomy space (if healing of the flap edges hasn’t occurred) with resultant IOP lowering.
FIGURE 27–6 Cataract incision made under a trabeculectomy flap should enter the anterior chamber 1 mm anterior to the anterior edge of the flap to avoid iris prolapse.
It is important to fill the anterior chamber, through the paracentesis, with balanced salt solution, before closing the conjunctiva. This will allow assessment of aqueous egress beneath the flap. If there is excess egress, additional sutures should be placed to further diminish flow. If there is a paucity of egress, flap sutures should be loosened and sometimes the sclerectomy has to be enlarged in size to increase flow. Although unreported, this surgeon finds that patients who are phakic have a greater tendency to develop a shallow or flat anterior chamber postoperatively than do pseudophakes or aphakes. Consequently, the former group usually gets more tightly tied flaps than either of the latter groups.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree