Peritoneal dialysis (PD) was the first successfully used modality of renal replacement therapy in acute kidney injury (AKI) patients. However, its use progressively declined after 1970 due to the greater convenience of acute hemodialysis, and it is now predominantly practiced in developing countries because of its lower cost and minimal infrastructure requirements. Recently, however, interest in using PD to manage selected AKI patients has been increasing (
Ghaffari, 2013b), and a meta-analysis suggests outcomes equivalent to those with HD (
Chionh, 2010).
I. INDICATIONS
A. Advantages. PD offers several advantages over hemodialysis in AKI. It is technically simple, with minimal infrastructure requirements and often lower cost. It may be the better option for the patient with difficult vascular access. Solute and water removal is gradual, with less potential for the development of disequilibrium syndrome, cardiovascular stress, and abrupt reductions in blood pressure. These potential benefits may, in turn, reduce the risk of renal and cardiac ischemia, fluid and electrolyte imbalance, and intracranial fluid shifts. No extracorporeal circulation is required, thus reducing the potential proinflammatory changes that can occur with the exposure of blood to synthetic tubing and membranes. Taken together, these factors could potentially be beneficial in permitting more rapid recovery of renal function.
Besides the classical indications (volume overload, electrolyte disorders, uremic symptoms, or acid-base disturbances), acute PD can also be used to maintain volume control in patients with congestive heart failure (CHF) functional class IV, to control hyper- or hypothermia, and to treat necrotizing pancreatitis with peritoneal lavage. Acute PD is increasingly being used in cases of advanced chronic kidney disease (CKD) presenting urgently with uremia or fluid overload, a scenario described as “urgent start PD.”
In the setting of natural disasters such as earthquakes, when several victims will develop AKI and when damage to
infrastructure make access to power, clean water, and facilities for water treatment challenging, PD can be an important and life-saving renal replacement modality.
Table 24.1 outlines the advantages and disadvantages of PD to treat patients with AKI.
B.
Limitations. PD is relatively contraindicated in patients with recent abdominal surgery, large abdominal hernias, adynamic ileus, intra-abdominal adhesions, peritoneal fibrosis, or peritonitis. Since volume and solute removal are slow and at times unpredictable, PD is not as safe and efficient as extracorporeal blood purification techniques for the treatment of certain emergencies, such as acute pulmonary edema, life-threatening hyperkalemia, and drug overdoses. The ability of PD to achieve adequate doses in hypercatabolic AKI has been a subject of controversy. Some authors have expressed concern over PD adequacy in these situations (
Phu, 2002). However, there are also reports of positive outcomes associated with PD in hypercatabolic AKI patients, especially when intensive PD regimens were used (
Chitalia, 2002;
Ponce, 2012b).
PD increases intra-abdominal pressure, which may lead to impaired diaphragm mobilization, decreasing pulmonary compliance and ventilation, and this may cause or worsen respiratory failure. However, patients on PD generally maintain their vital capacity and respiratory volume, and PD is seldom the cause of ventilation impairment in patients without pulmonary disease. Another possible limitation of PD in AKI is that
associated protein losses may aggravate malnutrition. Protein supplementation, either enteral or parenteral (1.5 g/kg per day) has been recommended for AKI patients on PD.
The high glucose concentrations in peritoneal dialysate may cause hyperglycemia, even in nondiabetic patients. This is easily correctable through intravenous, subcutaneous, or intraperitoneal administration of insulin. Peritonitis is a potential problem. Older studies reported a high frequency of peritonitis. However, with better catheter implantation techniques, improved connectology, and automated methods, the incidence has been reduced and the risk is similar to the incidence of infections with extracorporeal blood purification for AKI (
Ponce, 2011a).
II. TECHNICAL ASPECTS
A. Peritoneal access. Safe and efficient access to the peritoneal cavity is a crucial factor for PD success. For many years, bedsides insertion of a rigid catheter using a trocar was the standard technique to access the peritoneal cavity for acute PD. This technique is still used routinely in many parts of the world, but its use has declined with the introduction of simple procedures for insertion of a flexible, cuffed Tenckhoff catheter, which provides the optimal access for PD. Depending on availability, a single- or double-cuff Tenckhoff catheter—either straight or swan neck—can be used in AKI. The advantages of a Tenckhoff catheter over the rigid catheter include having a lower incidence of leakage, larger-diameter lumen, and side holes resulting in better dialysate flow rates, and less obstruction as well as a decreased incidence of peritonitis. Furthermore, the rigid catheters need to be removed after 3-5 days, while the flexible, cuffed catheters can be left in indefinitely. Thus, if the patient does not recover renal function, the catheter may be used for chronic dialysis. Of course, it may be necessary to use alternative catheters with a rigid stylet, or even improvised options such as nasogastric tubes or surgical drains, in resource-poor environments where flexible, cuffed catheters are not available or are too costly.
Tenckhoff catheters can be inserted under local anaesthesia at the bedside, in a designated treatment room, or in a surgical theater. In a patient with previous abdominal surgery, laparoscopic or open technique is preferred, and this will usually require an operating room and a surgeon. In patients without previous surgery, no method of insertion is proven to be superior to any other. Rather, the method of implantation should be based on local availability of skills, equipment, and consumables. The bedside insertion utilizes a modified Seldinger approach with a guidewire and “peel-away” sheath and is a method practised by many nephrologists. The catheter is inserted as a blind procedure and therefore this method should be avoided, if possible, in those patients who have a midline surgical scar or history to suggest intra-abdominal
adhesions. For details of catheter insertion mehods see
chapter 23.
B. PD solutions. Commercially prepared PD solutions are optimal because they have the advantage of minimizing the risks of errors in mixing fluids and of contamination, and of incorporating standardized and generally accepted connectology. Where these are not available because of logistical problems or costs, locally mixed fluids can be used, but sterile production and mixing of solutions as well as use of sterile connection devices are imperative. Such locally made PD fluids can be produced from physiological intravenous fluids by adding glucose and bicarbonate.
The composition of standard PD solutions is shown in
Table 22.1. Other commercial intravenous solutions that can relatively easily be converted into dialysis fluids include Ringer’s lactate, Hartmann’s solutions, half normal saline, and Plasmalyte B. Standard PD solutions generally use lactate as a buffer; this is converted to bicarbonate mainly through liver and muscle pyruvate dehydrogenase enzymes. In critically ill AKI patients (such as those with shock, poor tissue perfusion states, liver failure, etc.), there may be impaired conversion of lactate to bicarbonate, which can aggravate metabolic acidosis. In such patients, bicarbonate-containing PD solutions may be preferable. However, one small study randomized 20 AKI patients to treatment with either lactate- or bicarbonate-based PD and showed that while bicarbonate PD solution allowed better correction of metabolic acidosis and was associated with better hemodynamic stability, there were no differences in patient outcomes when compared to standard lactate solution (
Thongboonkerd, 2001).
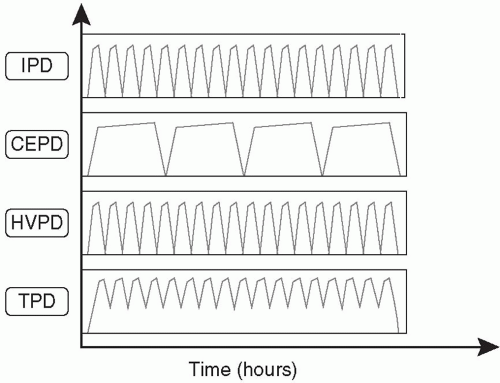
C. PD modalities. The process of dialysate instillation and removal can be automated with a PD cycler. The advantage of this system is that it can be set up by a trained staff member to reduce the risk of complications. It reduces nursing time as all cycles are automatic, and there is some suggestion that peritonitis is less likely. Automated cyclers have been used extensively to perform PD in AKI, particularly when high-volume peritoneal dialysis (HVPD) is used. However, in a resource-poor setting, cyclers may be unavailable or too expensive.
The choice of the type of PD to be utilized should be based on the experience of the medical and nursing team, available resources, the safety and efficacy of the technique, and the needs of the individual patient. An illustration of various techniques applicable to AKI is provided in
Figure 24.1 and
Table 24.2.
1.
Intermittent PD (IPD). This is the PD technique that historically has been most frequently used in AKI and it is still the most common, being routinely practiced in many parts of the world. Patients are treated for 48-72 hours, or occasionally longer, with rapid installation and drainage of fluid and a dwell time of 30-60 minutes. A trocar-style PD
catheter is traditionally used and removed after the dialysis treatment is completed, but Tenckhoff catheters are a better option and are increasingly available. Since the dialysis is interrupted when the catheter is removed, the weekly small-solute clearance is limited and might be inadequate in hypercatabolic, critically ill AKI patients. There are no recent large studies addressing this issue. Modeling suggests that IPD can deliver appropriate amounts of dialysis in a fairly broad range of clinical circumstances, depending on the degree of residual renal function (
Guest, 2012).