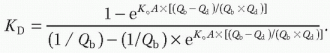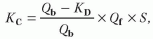Conventional diffusion-based dialysis modalities, including high-flux hemodialysis, are limited in their capacity to effectively remove larger-molecular-weight uremic toxins. Hemodiafiltration (HDF) is a modality that enhances convective solute transport of a broad range of uremic toxins known to be involved in uremia (
Vanholder, 2003) and may provide patients with a number of benefits, including improved outcomes.
I. DIFFUSION VERSUS CONVECTION-BASED CLEARANCES. Hemodialysis relies on diffusive transport. The rate of diffusion of the molecules is inversely proportional to the square root of their molecular weight. Larger molecules have a relatively low speed of diffusion and therefore a relatively slow clearance by hemodialysis. Convective transport depends on solvent drag, where molecules, regardless of their molecular weight, are transported across the membrane by bulk fluid flow. The extent to which the solute is taken across the membrane depends on the so-called sieving coefficient, which varies from 0 to 1.0. The sieving coefficient is different for different solutes and also depends on membrane characteristics. Convective transport markedly increases the removal of those middle- and large-sized molecules that are poorly cleared by diffusion-based therapies.
II. BASICS OF HEMODIAFILTRATION. HDF is a “hybrid” therapy combining within the same dialyzer module the two main solute transport mechanisms described above: diffusion and convection.
A.
Clearance due to diffusion and convection in HDF. The total clearance results from the sum of diffusive and convective clearances. For detailed equations, see
Table 17.1. Convective clearance for a given solute depends on the total ultrafiltered volume and the solute sieving coefficient for the membrane being used. The total ultrafiltered volume is the sum of the fluid removed during the treatment for the purpose of correcting extracellular fluid overload plus the volume of “replacement or substitution fluid” infused during the treatment for the purpose of enhancing convection.
B.
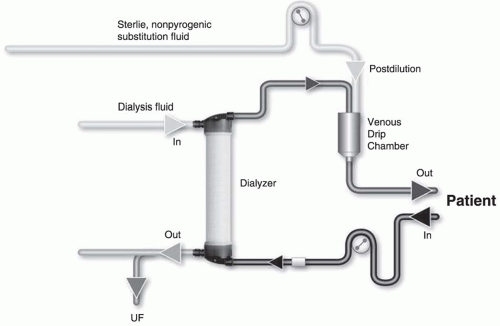
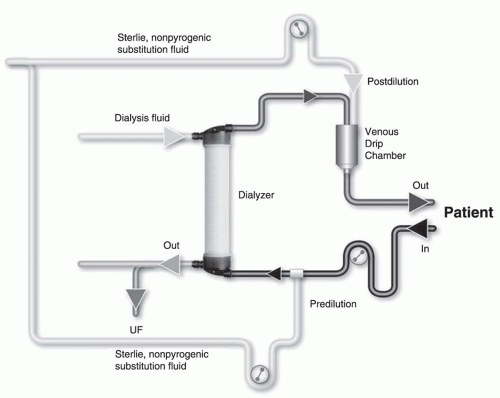
Substitution mode: Postdilution, predilution, and mixed dilution. Infusing the substitution fluid solution in postdilution mode (
Fig. 17.1) means that the fluid is added to the blood flow stream as it exits the hemodialyzer. This is the most efficient option for solute clearances (
Fig. 17.1) because there is no dilution of blood in the dialyzer, where convection is occurring. However, when blood flow rate is limited, or when adverse
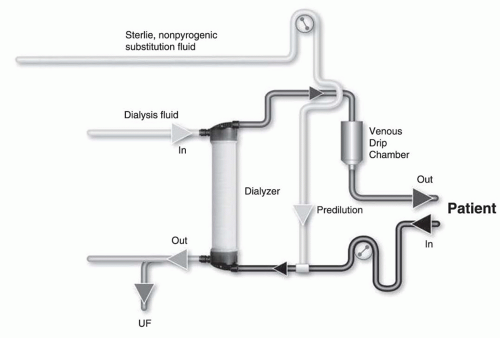
hemorheological conditions (such as high hemoglobin or high protein concentration) are present, or when the rate of substitution fluid infusion needs to be quite high, to avoid hemoconcentration in the filter, one can infuse all of the replacement fluid (predilution mode,
Fig. 17.2) or a part of it (mixed dilution,
Fig. 17.3) into the blood line upstream to the filter (
Pedrini, 2003). The predilution and mixed-dilution modes reduce solute clearance considerably because the concentration of toxins in the blood entering the dialyzer is diluted by the substitution fluid. Advantages and disadvantages of each substitution fluid infusion mode are given in
Table 17.2.
C. Technical issues
1. Vascular access. Patients treated with HDF require a vascular access capable of delivering an extracorporeal blood flow of at least 350-400 mL/min on a reliable basis.
2. High-flux hemodiafilter. The semipermeable membrane should have a high hydraulic permeability (KUF >50 mL per hour per mm Hg), high solute permeability (sieving coefficient for β2-microglobulin >0.6), and an optimal surface area of exchange (1.60-1.80 m2). Further, low internal blood resistance (internal fiber diameter >200 micrometers, sufficient number of fibers; length of fiber bundle <30 cm) is highly desirable to reduce hemoconcentration and facilitate ultrafiltration.
3.
Online production of substitution fluid. Outside of the United States, most dialysis machine manufacturers provide an upgrade option enabling direct production of substitution fluid for intravenous infusion from dialysis solution (
Blankestijn, 2010). Such online techniques enable provision of virtually unlimited amounts of sterile nonpyrogenic substitution fluid at a relatively low cost. All studies have shown that online production of substitution fluid is a safe, reliable, and economically viable solution for routine clinical application (
Canaud, 2000). This approach has gained the approval of all European regulatory bodies operating under the European Community label.
The production of sterile and nonpyrogenic dialysis fluid (ultrapure dialysate) is achieved by “cold sterilization” of the freshly prepared dialysis solution using dedicated sterilizing ultrafilters. The infusion module consists of an adjustable infusion pump that can be set to operate at 0-250 mL/minute. The ultrapure dialysate produced in this manner is then diverted by the infusion pump and passed through a second ultrafilter. The doubly filtered substitution fluid is then infused into the patient’s blood. The sterilizing ultrafilters are incorporated in the dialysis fluid paths and are disinfected in situ within the machine. They need to be replaced periodically according to defined criteria (either number of sessions or duration of use) to prevent loss of their endotoxin adsorption capacity.
4.
Water quality. Water used for convection-based therapies needs to comply with very stringent criteria of purity. Such high refinement in water purification has led to the concept of “ultrapure water”—virtually sterile and nonpyrogenic water. The overall target of this concept is to ensure both the chemical and microbiological purity of all fluids used. Technical aspects of water treatment systems and water distribution piping systems have been detailed elsewhere. The basic technical options required to produce ultrapure water consists of a pretreatment system (microfiltration, softeners, activated carbon, downstream microfiltration) that is followed by two reverse osmosis modules in series.
Ultrapurified water is delivered to dialysis machines via a distribution loop that ensures continuous recirculation of water. As already described, microbiological-quality dialysis fluid is derived from this chemically pure water by using downstream sterilizing ultrafilters incorporated within the dialysis machine.