Peptic Ulcer Disease
KEY CONCEPTS
![]() Patients with peptic ulcer disease (PUD) should reduce psychological stress, cigarette smoking, and nonsteroidal antiinflammatory drug (NSAID) use and avoid foods and beverages that exacerbate ulcer symptoms.
Patients with peptic ulcer disease (PUD) should reduce psychological stress, cigarette smoking, and nonsteroidal antiinflammatory drug (NSAID) use and avoid foods and beverages that exacerbate ulcer symptoms.
![]() Eradication is recommended for all Helicobacter pylori–positive patients, especially those patients with an active ulcer, a documented history of a prior ulcer, or a history of ulcer-related complications.
Eradication is recommended for all Helicobacter pylori–positive patients, especially those patients with an active ulcer, a documented history of a prior ulcer, or a history of ulcer-related complications.
![]() The selection of an H. pylori eradication regimen should be based on efficacy, safety, antibiotic resistance, cost, and the likelihood of medication adherence. Treatment should be initiated with a proton pump inhibitor (PPI)–based three-drug regimen. If a second course of H. pylori therapy is required, the regimen should contain different antibiotics.
The selection of an H. pylori eradication regimen should be based on efficacy, safety, antibiotic resistance, cost, and the likelihood of medication adherence. Treatment should be initiated with a proton pump inhibitor (PPI)–based three-drug regimen. If a second course of H. pylori therapy is required, the regimen should contain different antibiotics.
![]() PPI cotherapy reduces the risk of NSAID-related gastric and duodenal ulcers and is at least as effective as recommended dosages of misoprostol and superior to the histamine-2 receptor antagonists (H2RAs).
PPI cotherapy reduces the risk of NSAID-related gastric and duodenal ulcers and is at least as effective as recommended dosages of misoprostol and superior to the histamine-2 receptor antagonists (H2RAs).
![]() Standard PPI dosages and a nonselective NSAID are as effective as a selective cyclooxygenase-2 (COX-2) inhibitor in reducing the risk of NSAID-induced ulcers and upper GI complications.
Standard PPI dosages and a nonselective NSAID are as effective as a selective cyclooxygenase-2 (COX-2) inhibitor in reducing the risk of NSAID-induced ulcers and upper GI complications.
![]() Patients with PUD, especially those receiving H. pylori eradication or misoprostol cotherapy, require patient education regarding their disease and drug treatment to successfully achieve a positive therapeutic outcome.
Patients with PUD, especially those receiving H. pylori eradication or misoprostol cotherapy, require patient education regarding their disease and drug treatment to successfully achieve a positive therapeutic outcome.
![]() The recommended treatment for severe peptic ulcer bleeding after appropriate endoscopic treatment is the IV administration of a PPI loading dose followed by a 72-hour continuous infusion with a goal of maintaining an intragastric pH of 6 or greater.
The recommended treatment for severe peptic ulcer bleeding after appropriate endoscopic treatment is the IV administration of a PPI loading dose followed by a 72-hour continuous infusion with a goal of maintaining an intragastric pH of 6 or greater.
![]() Critically ill patients at the highest risk of developing stress-related mucosal bleeding (SRMB) who require prophylactic drug therapy include those with respiratory failure on mechanical ventilation or those with coagulopathy.
Critically ill patients at the highest risk of developing stress-related mucosal bleeding (SRMB) who require prophylactic drug therapy include those with respiratory failure on mechanical ventilation or those with coagulopathy.
![]() There are limited data to support the selection of a PPI over an IV H2RA for SRMB prophylaxis. The decision should be based on appropriate individual patient characteristics (e.g., nothing by mouth, presence of nasogastric tube, renal failure).
There are limited data to support the selection of a PPI over an IV H2RA for SRMB prophylaxis. The decision should be based on appropriate individual patient characteristics (e.g., nothing by mouth, presence of nasogastric tube, renal failure).
PEPTIC ULCER DISEASE
Acid-related diseases (gastritis, erosions, and peptic ulcer) of the upper GI tract require gastric acid for their formation.1–3 Peptic ulcer disease (PUD) differs from gastritis and erosions in that ulcers typically extend deeper into the muscularis mucosa.1 There are three common forms of peptic ulcers: Helicobacter pylori positive, nonsteroidal antiinflammatory drug (NSAID) induced, and stress ulcers (Table 20-1). The term stress-related mucosal damage (SRMD) is preferred to stress ulcer or stress gastritis, because the mucosal lesions range from superficial gastritis and erosions to deep ulcers.
TABLE 20-1 Comparison of Common Forms of Peptic Ulcer
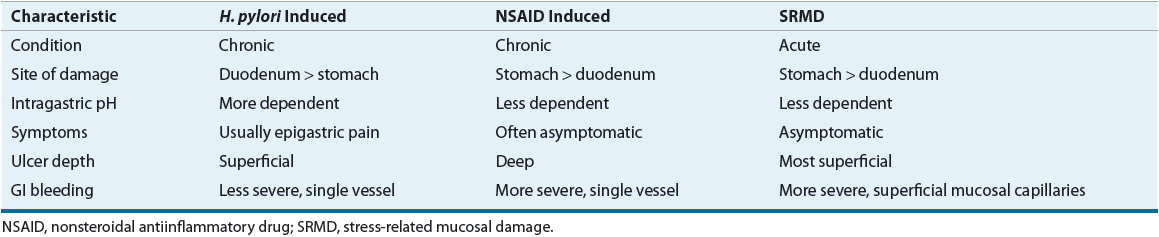
H. pylori–positive and NSAID-induced ulcers are chronic peptic ulcers that differ in etiology, clinical presentation, and tendency to recur (see Table 20-1). These ulcers develop most often in the stomach and duodenum of ambulatory patients (Fig. 20-1). Occasionally, ulcers develop in the esophagus, jejunum, ileum, or colon. The natural course of chronic PUD is characterized by frequent ulcer recurrence. The most important factors that influence ulcer recurrence are H. pylori infection and NSAID use. Other factors include cigarette smoking, alcohol use, ulcer-related complications, gastric acid hypersecretion, and patient noncompliance. The cause of ulcer recurrence is most likely multifactorial.
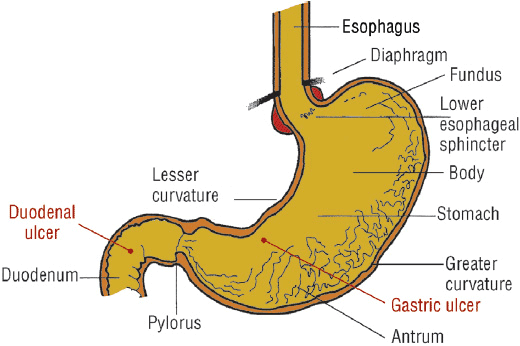
FIGURE 20-1 Anatomic structure of the stomach and duodenum and most common locations of gastric and duodenal ulcers.
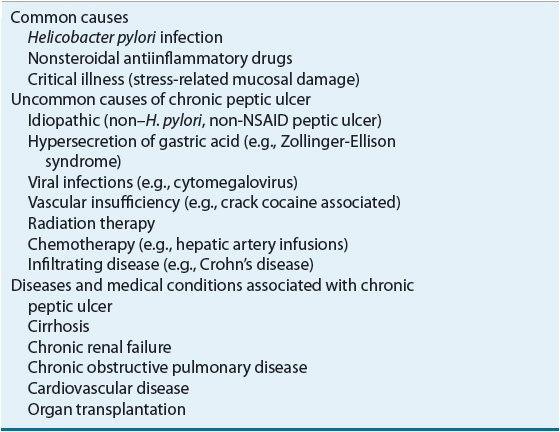
Peptic ulcers are also associated with Zollinger-Ellison syndrome (ZES), radiation, chemotherapy, vascular insufficiency, and other chronic diseases (Table 20-2).1,3 Although a strong association exists between chronic pulmonary diseases, chronic renal failure, and cirrhosis, the pathophysiologic mechanisms of these associations remain unclear.1 In contrast, SRMD occurs primarily in the stomach in critically ill patients (see Table 20-1).1
TABLE 20-2 Potential Causes of Peptic Ulcer
This chapter focuses on chronic PUD associated with H. pylori and NSAIDs. A brief discussion of ZES and upper GI bleeding related to PUD and SRMD is included.
EPIDEMIOLOGY
The epidemiology of PUD is complicated and difficult to estimate given the variability in the prevalence of H. pylori infection, NSAID use, and cigarette smoking as well as the various methods used to detect ulcers, for example, endoscopy, radiology, symptoms, or complications.1,4 The prevalence and incidence of PUD in the United States also reflects improvements in drug therapy, the dramatic shift to ambulatory management, and changes in the criteria and coding system for mortality and hospitalization data.1 Recent trends suggest a shift from predominance in men to a similar occurrence in men and women with increasing rates of disease in older individuals and a decrease in the younger population.1,4 Despite a modest decline in mortality, hospitalizations, and office visits, PUD remains one of the most common GI diseases, resulting in impaired quality of life, work loss, and high-cost medical care.
ETIOLOGY AND RISK FACTORS
Most peptic ulcers occur in the presence of acid and pepsin when H. pylori, NSAIDs, or other factors (see Table 20-2) disrupt the normal mucosal defense and healing mechanisms.1 Hypersecretion of acid is the primary pathogenic mechanism in hypersecretory states such as ZES.3 Benign gastric ulcers can occur anywhere in the stomach, although most are located on the lesser curvature, just distal to the junction of the antral and acid-secreting mucosa (see Fig. 20-1). Most duodenal ulcers occur in the first part of the duodenum (duodenal bulb).
H. pylori
H. pylori infection causes chronic gastritis in infected individuals and is causally linked to PUD, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer (Fig. 20-2).1,2,5–8 The majority of infected individuals remain asymptomatic, but 10% to 20% will develop PUD during their lifetime and about 1% will develop gastric cancer.1,2,8 Host-specific cofactors and H. pylori strain variability play an important role in the pathogenesis of PUD and gastric cancer.2,8 Although an association between H. pylori and PUD bleeding is less clear, there is evidence that eradication of H. pylori decreases recurrent bleeding.5,9 No specific link has been established between H. pylori and dyspepsia, nonulcer dyspepsia (NUD), or gastroesophageal reflux disease (GERD).5,9–11 However, some patients with dyspepsia and NUD may have symptom improvement from H. pylori eradication.5,9 Although eradication of H. pylori may worsen GERD symptoms in some patients, eradication should not be withheld.5,10 An association between H. pylori infection and iron deficiency anemia has been established, but cause and effect have not been proven, and whether H. pylori eradication is beneficial is uncertain.5,11 There are insufficient data to support a link between H. pylori and extragastric manifestations including cardiovascular, hematologic, respiratory, hepatobiliary, and neurologic diseases.5,11
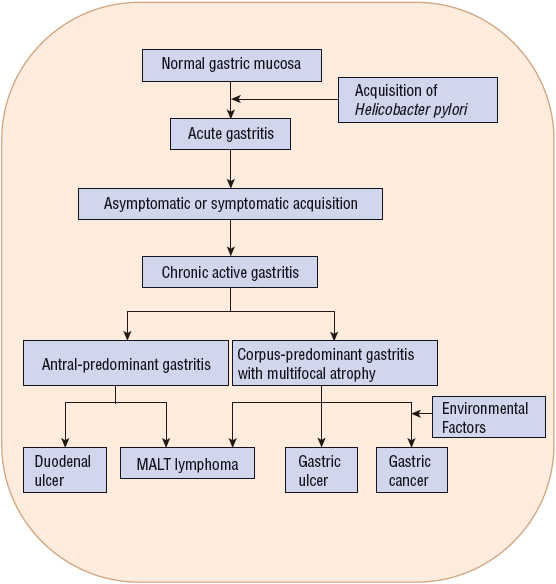
FIGURE 20-2 The natural history of Helicobacter pylori infection in the pathogenesis of gastric ulcer and duodenal ulcer, mucosaassociated lymphoid tissue (MALT) lymphoma, and gastric cancer.
The prevalence of H. pylori varies by geographic location, socioeconomic conditions, ethnicity, and age. In developing countries, H. pylori prevalence is more common than in industrialized countries and correlates with lower socioeconomic levels.2,5,6 The prevalence of H. pylori in the United States is 30% to 40% but is much higher in individuals over 60 years (50% to 60%) than in children under 12 years (10% to 15%) of age.2,5 Although most individuals in the United States acquire H. pylori in childhood, the rate of acquisition in children is declining and most likely will continue to fall as a consequence of improved socioeconomic conditions.2 Whites are infected with H. pylori less frequently than African Americans and Hispanic persons, but this is thought to be related to lower socioecomonic status and living conditions. Infection rates do not differ with gender or smoking status.
The most common route of H. pylori transmission is person to person by either gastro–oral (vomitus) or fecal–oral (diarrhea) contact that occurs primarily during childhood.2 Members of the same household are likely to become infected when someone in the same household is infected.2 H. pylori can also be transmitted by the use of inadequately sterilized endoscopes.
Nonsteroidal Antiinflammatory Drugs
NSAIDs (Table 20-3), including both prescription and nonprescription medications, are widely used in the United States, particularly in individuals over 60 years of age, to treat chronic pain and inflammation.1 Low-dose aspirin is used for cardiovascular and cerebrovascular risk reduction.1,12–14 There is overwhelming evidence linking chronic NSAID (including aspirin) use to a variety of upper GI tract injuries.1,12–16 NSAIDs cause superficial (topical) mucosal damage consisting of petechiae (intramucosal hemorrhages) within minutes of ingestion, and progress to erosions with continued use.1 These lesions typically heal within a few days and rarely cause ulcers or acute upper GI bleeding. Gastroduodenal ulcers develop in about 25% of chronic NSAID users with continued use.12 Gastric ulcers are most common, occur primarily in the antrum, and are of greater concern because of their potential to cause ulcer-related upper GI complications (see Table 20-1). As many as 2% to 4% of patients with an NSAID ulcer will bleed or perforate.12 Each year, NSAIDs account for at least 100,000 hospitalizations and between 7,000 and 10,000 deaths in the United States.12 NSAID-induced ulcers occur less frequently in the esophagus, small bowel, and colon.16,17 How NSAIDs damage the lower GI tract is unclear, but the enteropathy is associated with lower GI bleeding.
TABLE 20-3 Selected Nonsteroidal Antiinflammatory Drugs (NSAIDs) and Cyclooxygenase-2 (COX-2) Inhibitors

Table 20-4 lists the risk factors associated with NSAID-induced ulcers and upper GI complications. Combinations of factors confer an additive risk.12–16,18 Advanced age is an independent risk factor, and the incidence of NSAID-induced ulcers increases linearly with the age of the patient.1 The high incidence of ulcer complications in older individuals may be explained by age-related changes in gastric mucosal defense. The relative risk of NSAID complications is increased for patients with a previous peptic ulcer and may be as high as 14-fold in those with a history of an ulcer-related complication.1,16 Although the risk of ulcer complications is greatest during the first few months after initiating continuous NSAID therapy, it does not vanish with long-term treatment.12
TABLE 20-4 Risk Factors Associated with Nonsteroidal Antiinflammatory Drug (NSAID)–Induced Ulcers and Upper GI Complicationsa
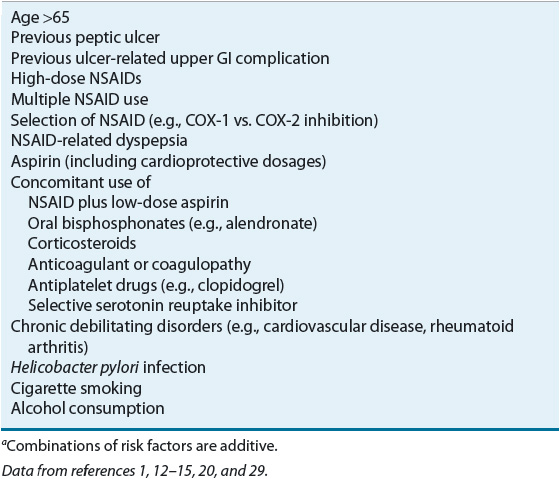
NSAID ulcers and related complications are dose dependent, but may occur with low doses of nonprescription NSAIDs and low cardioprotective dosages of aspirin (81 to 325 mg/day).1,12–16 Factors such as NSAID potency, longer duration of effect, and a greater propensity to inhibit cyclooxygenase-1 (COX-1) versus cyclooxygenase-2 (COX-2) isoenzymes are associated with increased risk (see Table 20-3).1,16,18,19 NSAID-related dyspepsia, in itself, does not correlate directly with mucosal injury or clinical events. However, new-onset dyspepsia, changes in severity, or dyspepsia not relieved by antiulcer medications may suggest an ulcer or ulcer complication.1 Nonacetylated salicylates (e.g., salsalate) may be associated with decreased GI toxicity.1 Buffered or enteric-coated aspirin confers no added protection from upper GI events.13
NSAID ulcer and GI complication risk are increased with the use of multiple NSAIDs or the concomitant use of low-dose aspirin, oral bisphosphonates, corticosteroids, anticoagulants, antiplatelet drugs, and selective serotonin reuptake inhibitors.1,12–16,20 The risk of an ulcer-related GI complication is greater when an NSAID or COX-2 inhibitor (see Table 20-3) is coadministered with low-dose aspirin than when either drug is taken alone.1,13,16 The NSAID may also reduce the antiplatelet effects of aspirin, although NSAIDs vary in their effects on platelet function.13–15 Corticosteroids, when used alone, do not potentiate the risk of ulcer or complications, but the relative risk is increased twofold in corticosteroid users who are also taking concurrent NSAIDs.1,16 The relative risk of GI bleeding increases up to 20-fold when NSAIDs are taken concomitantly with anticoagulants (e.g., warfarin) and up to 6-fold with the concurrent use of serotonin reuptake inhibitors.16,17 When clopidogrel is taken in combination with aspirin, an NSAID, or an anticoagulant, the risk of GI bleeding is increased compared with when these agents are taken alone.13,15,16 Even when prescribed as monotherapy, clopidogrel increases the risk of rebleeding for patients with a history of a bleeding ulcer.13,15,16
H. pylori and NSAIDs act independently to increase ulcer risk and ulcer-related bleeding and appear to have additive effects.5,16 Thus, the incidence of peptic ulcer is higher in H. pylori–positive NSAID users. Whether H. pylori infection is actually a risk factor for NSAID ulcers remains controversial.1,5,16 However, eradication is reported to reduce the incidence of peptic ulcer if undertaken prior to starting the NSAID but does not reduce the risk for patients who were previously taking an NSAID.1,5,16
Cigarette Smoking
Epidemiologic evidence links cigarette smoking to PUD, but it is uncertain whether smoking causes peptic ulcers.1 Ulcer risk is proportional to the number of cigarettes smoked and is modest when fewer than 10 cigarettes are smoked per day. Cigarette smoking impairs ulcer healing, promotes ulcer recurrence, and increases ulcer risk.1 The exact mechanism by which cigarette smoking contributes to PUD remains unclear. Possible mechanisms include mucosal ischemia, inhibition of pancreatic bicarbonate secretion, and increases in gastric acid secretion, but these effects are inconsistent. Whether nicotine or other components of smoke are responsible for these physiologic alterations is unknown.
Psychological Stress
The importance of psychological factors in the pathogenesis of PUD remains controversial.1 Clinical observation suggests that ulcer patients are adversely affected by stressful life events. However, results from controlled trials are conflicting and have failed to document a cause-and-effect relationship.1 Emotional stress may induce behavioral risks such as smoking and the use of NSAIDs or alter the inflammatory response or resistance to H. pylori infection. The role of stress and how it affects PUD is complex and probably multifactorial.
Dietary Factors
The role of diet and nutrition in PUD is uncertain.1 Coffee, tea, carbonated beverages, beer, milk, and spices may cause dyspepsia but do not increase the risk for PUD. Beverage restrictions and bland diets do not alter the frequency of ulcer recurrence. Although caffeine is a gastric acid stimulant, constituents in decaffeinated coffee or tea, caffeine-free carbonated beverages, beer, and wine may also increase gastric acid secretion. In high concentrations, alcohol ingestion is associated with acute gastric mucosal damage and upper GI bleeding; however, there is insufficient evidence to confirm that alcohol causes ulcers.1
PATHOPHYSIOLOGY
A physiologic imbalance between aggressive (gastric acid and pepsin) and protective (mucosal defense and repair) factors remains an important issue in the pathophysiology of gastric and duodenal ulcers.1,21 Gastric acid is secreted by the parietal cells, which contain receptors for histamine, gastrin, and acetylcholine.21 Acid (as well as H. pylori infection and NSAID use) is an independent factor that contributes to the disruption of mucosal integrity.1 Increased acid secretion has been observed for patients with duodenal ulcers and may be a consequence of H. pylori infection.2,22 Patients with ZES (described in Zollinger-Ellison Syndrome below) have profound gastric acid hypersecretion resulting from a gastrin-producing tumor.3 In contrast, patients with gastric ulcer usually have normal or reduced rates of acid secretion (hypochlorhydria).
Acid secretion is expressed as the amount of acid secreted under basal or fasting conditions, basal acid output (BAO); after maximal stimulation, maximal acid output (MAO); or in response to a meal.21 Basal, maximal, and meal-stimulated acid secretion varies according to time of day and the individual’s psychological state, age, gender, and health status. The BAO follows a circadian rhythm, with the highest acid secretion occurring at night and the lowest in the morning. An increase in the BAO:MAO ratio suggests a basal hypersecretory state such as ZES. A review of gastric acid secretion and its regulation can be found elsewhere.21
Pepsin is an important cofactor that plays a role in the proteolytic activity involved in ulcer formation.21 Pepsinogen, the inactive precursor of pepsin, is secreted by the chief cells located in the gastric fundus (see Fig. 20-1). Pepsin is activated by acid pH (optimal pH of 1.8 to 3.5), reversibly inactivated at pH 4, and irreversibly destroyed at pH 7.
Mucosal defense and repair mechanisms (mucus and bicarbonate secretion, intrinsic epithelial cell defense, and mucosal blood flow) protect the gastroduodenal mucosa from noxious endogenous and exogenous substances.1,21 The viscous nature and near-neutral pH of the mucus–bicarbonate barrier protect the stomach from the acidic contents in the gastric lumen. Mucosal repair after injury is related to epithelial cell restitution, growth, and regeneration. The maintenance of mucosal integrity and repair is mediated by the production of endogenous prostaglandins (PGs). The term cytoprotection is often used to describe this process, but mucosal defense and mucosal protection are more accurate terms, as PGs prevent deep mucosal injury and not superficial damage to individual cells. Gastric hyperemia and increased PG synthesis characterize adaptive cytoprotection, the short-term adaptation of mucosal cells to mild topical irritants. This phenomenon enables the stomach to initially withstand the damaging effects of irritants. Alterations in mucosal defense that are induced by H. pylori or NSAIDs are the most important cofactors in the formation of peptic ulcers.
H. pylori
H. pylori is a spiral-shaped, pH-sensitive, gram-negative, microaerophilic bacterium that resides between the mucus layer and surface epithelial cells in the stomach, or any location where gastric-type epithelium is found.2,22 The combination of its spiral shape and flagellum permits it to move from the lumen of the stomach, where the pH is low, to the mucus layer, where the local pH is neutral. H. pylori produces large amounts of urease, which hydrolyzes urea in the gastric juice and converts it to ammonia and carbon dioxide.2 The local buffering effect of ammonia creates a neutral microenvironment within and surrounding the bacterium, protecting it from the lethal effect of gastric acid. H. pylori also produces acid-inhibitory proteins, which allow it to adapt to the low-pH environment of the stomach.2
H. pylori binds to specific regions within the stomach. It attaches to gastric-type epithelium by adherence pedestals, which prevent the organism from being shed during cell turnover and mucus secretion.2 Colonization of the antrum and corpus (body) of the stomach is associated with gastric ulcer and cancer.1,2,22 Antral organisms colonize gastric metaplastic tissue (gastric tissue that develops in the duodenum secondary to changes in gastric acid or bicarbonate secretion) leading to duodenal ulcer (see Fig. 20-2).1,2 Although H. pylori causes chronic gastric mucosal inflammation in all infected individuals, only a minority actually develop an ulcer or gastric cancer.1,2,8 The difference in the diverse clinical outcomes is related to variations in bacterial pathogenicity and host susceptibility.2,22
Mucosal injury is produced by (a) elaborating bacterial enzymes (urease, lipases, and proteases), (b) adherence, and (c) H. pylori virulence factors.2,22 Lipases and proteases degrade gastric mucus, ammonia produced by urease may be toxic to gastric epithelial cells, and bacterial adherence enhances the uptake of toxins into gastric epithelial cells. H. pylori induces gastric inflammation by altering the host inflammatory response and damaging epithelial cells directly by cell-mediated immune mechanisms or indirectly by activated neutrophils or macrophages attempting to phagocytose bacteria or bacterial products.2,22 However, H. pylori strains are genetically diverse and account for differences in adaptation within the human host. Two of the most important are cytotoxin-associated gene protein (CagA) and vacuolating cytotoxin (VacA). About 60% of H. pylori strains in the United States possess CagA, but CagA-positive strains increase the risk for severe PUD, gastritis, and gastric cancer compared with CagA-negative strains.2,22 The VacA gene codes for the VacA cytotoxin, a vacuolating toxin. Although VacA is present in most H. pylori strains, strains vary in cytotoxicity and increased risk for peptic ulcer and gastric cancer.2,22 Host polymorphisms are important markers of disease susceptibility and may identify high-risk patients.2,22 Polymorphisms of interleukin (IL)-1β and its receptor antagonist, as well as tumor necrosis factor-α (TNF-α) and IL-10, may be associated with increased gastric acid secretion and duodenal ulcer or acid suppression and gastric cancer.2,22
Nonsteroidal Antiinflammatory Drugs
NSAIDs, including aspirin (see Table 20-3), cause gastric mucosal damage by two important mechanisms: (a) direct or topical irritation of the gastric epithelium and (b) systemic inhibition of endogenous mucosal PG synthesis.1,13 Although the onset of injury is initiated topically by the acidic properties of many of the NSAIDs, systemic inhibition of the protective PGs limits the ability of the mucosa to defend itself against injury and thus plays the predominant role in the development of gastric ulcer.1,13
Topical irritant properties are predominantly associated with acidic NSAIDs (e.g., aspirin) and their ability to decrease the hydrophobicity of the mucous gel layer in the gastric mucosa. Most non-aspirin NSAIDs have topical irritant effects, but aspirin is the most damaging. Although NSAID prodrugs, enteric-coated aspirin tablets, salicylate derivatives, and parenteral or rectal preparations are associated with less acute topical gastric mucosal injury, they can cause ulcers and related GI complications as a result of their systemic inhibition of endogenous PGs.1
Cyclooxygenase (COX) is the rate-limiting enzyme in the conversion of arachidonic acid to PGs and is inhibited by NSAIDs (Fig. 20-3). Two similar COX isoforms have been identified: COX-1 is found in most body tissue, including the stomach, kidney, intestine, and platelets; COX-2 is undetectable in most tissues under normal physiologic conditions, but its expression can be induced during acute inflammation and arthritis (Fig. 20-4).1,13 COX-1 produces protective PGs that regulate physiologic processes such as GI mucosal integrity, platelet homeostasis, and renal function. COX-2 is induced (unregulated) by inflammatory stimuli such as cytokines and produces PGs involved with inflammation, fever, and pain. It is also constitutionally expressed in organs such as the brain, kidney, and reproductive tract. Adverse effects (e.g., GI or renal toxicity) of NSAIDs are primarily associated with the inhibition of COX-1, whereas antiinflammatory actions result primarily from NSAID inhibition of COX-2.1,13
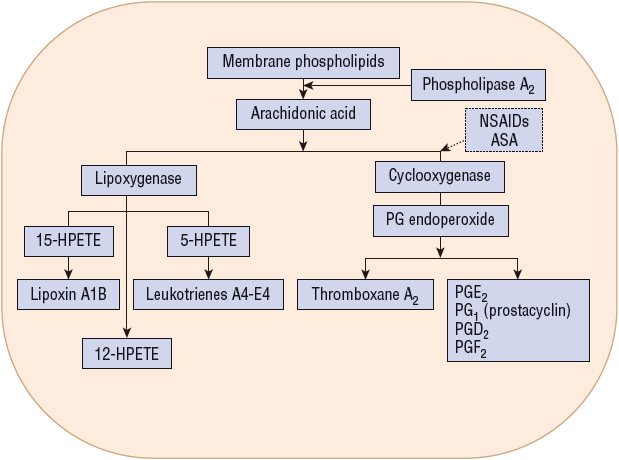
FIGURE 20-3 Metabolism of arachidonic acid after its release from membrane phospholipids. Broken arrow indicates inhibitory effects. (ASA, aspirin; HPETE, hydroperoxyeicosatetraenoic acid; NSAIDs, nonsteroidal antiinflammatory drugs; PG, prostaglandin.)
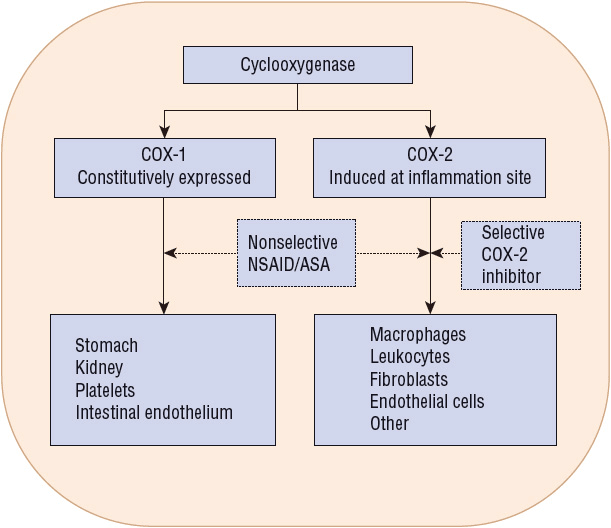
FIGURE 20-4 Tissue distribution and actions of cyclooxygenase (COX) isoenzymes. Nonselective nonsteroidal antiinflammatory drugs (NSAIDs) including aspirin (ASA) inhibit COX-1 and COX-2 to varying degrees; COX-2 inhibitors inhibit only COX-2. Broken arrow indicates inhibitory effects.
The COX-1-to-COX-2 inhibitory ratio determines the relative GI toxicity of a specific NSAID. Nonselective NSAIDs, including aspirin (see Table 20-3), inhibit both COX-1 and COX-2 to varying degrees and are associated with an increased propensity to cause gastric ulcers.1,13 In contrast, the selective COX-2 inhibitors are associated with a reduction in ulcers and related GI complications, but the benefit of celecoxib is less than that of rofecoxib and valdecoxib (see Table 20-3). The addition of aspirin to a selective COX-2 inhibitor reduces its ulcer-sparing benefit and increases ulcer risk.1,13 Aspirin and nonaspirin NSAIDs irreversibly inhibit platelet COX-1, resulting in decreased platelet aggregation and prolonged bleeding times, thereby increasing the potential for upper and lower GI bleeding.1,13,15 Coadministration of selected NSAIDs may reduce the antiplatelet effects of aspirin.13–15 Clopidogrel and other medications that impair angiogenesis do not cause ulcers, per se, but may impair healing of gastric erosions leading to ulceration.13,15
Complications
Upper GI bleeding, perforation, and obstruction occur with H. pylori–associated and NSAID-induced ulcers and constitute the most serious, life-threatening complication of chronic PUD.1,23 Bleeding is caused by the erosion of an ulcer into an artery. It may be occult (hidden) and insidious or may present as melena (black-colored stools) or hematemesis (vomiting of blood). The use of NSAIDs (especially in older adults) is the most important risk factor for upper GI bleeding. Deaths occur primarily in patients who continue to bleed or in those patients who rebleed after the initial bleeding has stopped (see Upper GI Bleeding below).
Ulcer-related perforation into the peritoneal cavity is generally considered a surgical emergency.1,23 About one third to one half of perforated ulcers are associated with the use of NSAIDs, with the highest mortality reported in the elderly.23 The pain of perforation is usually sudden, sharp, and severe, beginning first in the epigastrium, but quickly spreading over the entire abdomen. Most patients experience ulcer symptoms prior to perforation. However, older patients who experience perforation in association with NSAID use may be asymptomatic. Penetration occurs when an ulcer burrows into an adjacent structure (pancreas, biliary tract, or liver) rather than opening freely into a cavity.
Gastric outlet obstruction is related to mechanical obstruction caused by scarring, muscular spasm, or edema of the duodenal bulb usually resulting from chronic ulceration.1,23 Symptoms occur over several months and include early satiety, bloating, anorexia, nausea, vomiting, and weight loss. Perforation, penetration, and gastric outlet obstruction occur most often with long-standing PUD.
Treatment of PUD has improved so that even the most virulent ulcers can be managed with medication. Intractability to drug therapy is an infrequent manifestation of PUD and an infrequent indication for surgery.
CLINICAL PRESENTATION
The clinical presentation of PUD varies depending on the severity of epigastric pain and the presence of complications (Table 20-5).1 Ulcer-related pain in duodenal ulcer often occurs 1 to 3 hours after meals and is usually relieved by food, but this is variable. In gastric ulcer, food may precipitate or accentuate ulcer pain. Antacids usually provide immediate pain relief in most ulcer patients. Pain usually diminishes or disappears during treatment; however, recurrence of epigastric pain after healing often suggests an unhealed or recurrent ulcer.
TABLE 20-5 Clinical Presentation of Peptic Ulcer Disease

The presence or absence of epigastric pain does not define an ulcer.1 Ulcer healing does not necessarily render the patient asymptomatic. Why symptoms remain is unclear, but it may relate to sensitization of afferent nerves in response to mucosal injury.1 Conversely, the absence of pain does not preclude an ulcer diagnosis especially in the elderly who may present with a “silent” ulcer complication. The reasons for this are unclear, but may relate to differences in the way the elderly perceive pain or the analgesic effect of NSAIDs.
Dyspepsia in itself is of little clinical value when assessing subsets of patients who are most likely to have an ulcer. Patients taking NSAIDs often report dyspepsia, but dyspeptic symptoms do not directly correlate with an ulcer. Individuals with dyspeptic symptoms may have either uninvestigated (no upper endoscopy) or investigated (underwent upper endoscopy) dyspepsia. If an ulcer is not confirmed in a patient with ulcer-like symptoms at the time of endoscopy, the disorder is referred to as NUD.9 Ulcer-like symptoms may occur in the absence of peptic ulceration in association with H. pylori gastritis or duodenitis. There is no one sign or symptom that differentiates between H. pylori–positive and NSAID-induced ulcer.
DIAGNOSIS
Routine laboratory tests are not helpful in establishing the diagnosis of PUD (see Table 20-5).1
Tests for H. pylori
The diagnosis of H. pylori infection can be made using endoscopic or nonendoscopic tests (Table 20-6).2,5,24 The tests that require upper endoscopy are invasive, more expensive, and usually require a mucosal biopsy for histology, culture, or detection of urease activity. At least four tissue samples are taken from specific areas of the stomach, as patchy distribution of H. pylori infection can lead to false-negative results. Because certain medications may decrease the sensitivity of rapid urease test, antibiotics and bismuth salts should be withheld for 4 weeks and proton pump inhibitors (PPIs) for 1 to 2 weeks prior to endoscopic testing.2,5 If the patient has been taking these medications, then a gastric biopsy for histology should be performed.5
TABLE 20-6 Tests for Detection of Helicobacter pylori
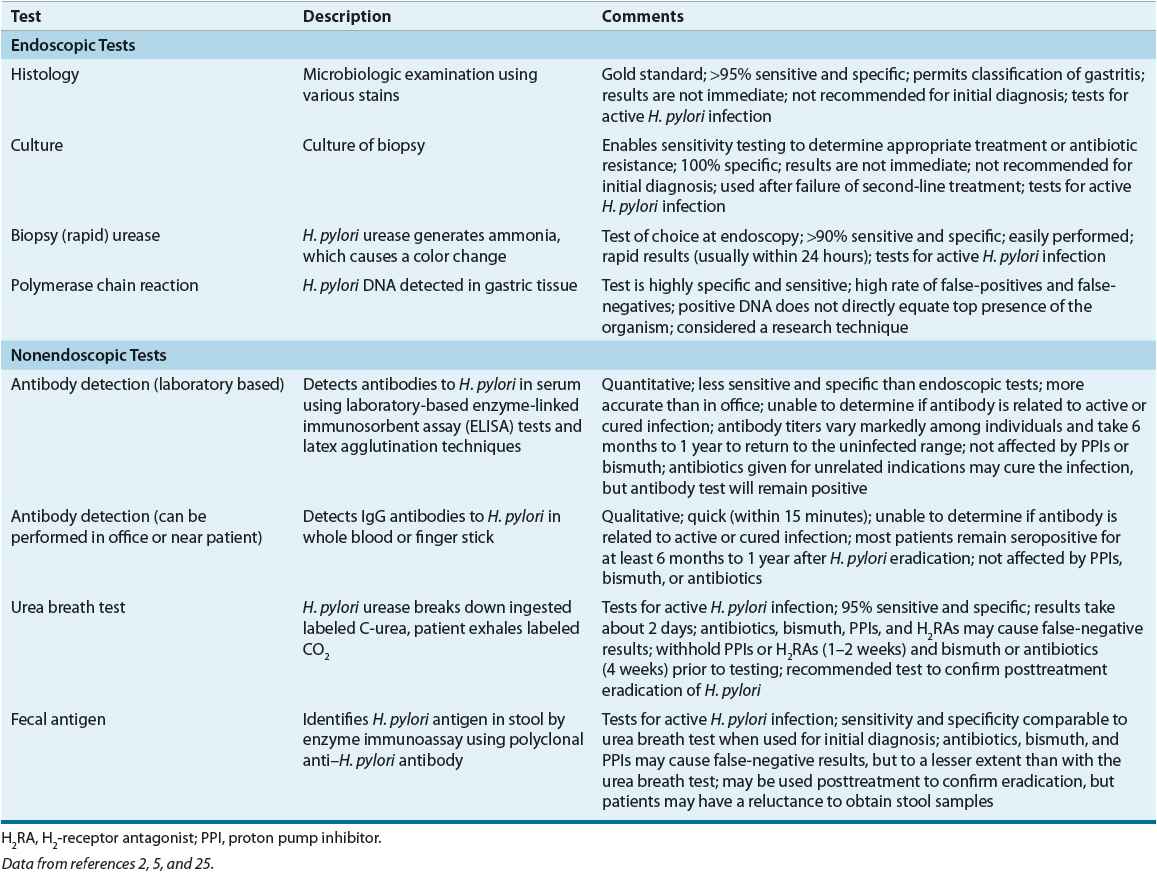
Two types of nonendoscopic tests are available: tests that identify active infection and tests that detect antibodies (see Table 20-6). Antibody tests do not differentiate between active infection and previously eradicated H. pylori. The nonendoscopic tests include the urea breath test (UBT), serologic antibody detection tests, and the fecal antigen test. These tests are less invasive, more convenient, and less expensive than the endoscopic tests.2,5,24
The UBT is the most accurate noninvasive test and is based on H. pylori urease activity.5 The 13Carbon (nonradioactive isotope) and 14Carbon (radioactive isotope) tests require that the patient ingest radiolabeled urea, which is then hydrolyzed by H. pylori (if present in the stomach) to ammonia and radiolabeled bicarbonate. The radiolabeled bicarbonate is absorbed in the blood and excreted in the breath. A mass spectrometer is used to detect 13Carbon, whereas 14Carbon is measured using a scintillation counter. The fecal antigen test is less expensive and easier to perform than the UBT, and may be useful in children.
Serologic tests are a cost-effective alternative for the initial diagnosis of H. pylori infection in the untreated patient.2,5 Antibodies to H. pylori usually develop about 3 weeks after infection and remain present after successful eradication.5 Therefore, serology should not be used to confirm H. pylori eradication.2,5 Office-based tests are less expensive, widely available, and provide rapid results, but the results are less accurate and more variable than the laboratory-based tests. Salivary and urine antibody tests are under investigation.2
Testing for H. pylori is only recommended if eradication is planned. Serologic antibody testing is a reasonable choice if endoscopy is not planned. The diagnostic accuracy of H. pylori tests for patients with an active bleeding ulcer has been questioned because of the potential for false-negative results. However, endoscopic biopsy-based tests such as the rapid urease test have a high degree of specificity in these patients (see Peptic Ulcer–Related Bleeding below).5
Confirmation of eradication is indicated posttreatment of active ulcers, previous ulcers, MALT lymphoma, endoscopic resection of gastric cancer, and uninvestigated dyspepsia, but routine testing for all patients is neither cost-effective nor practical.5 The decision to test posttreatment should be patient-specific and take into consideration the patient’s diagnosis, age, and ulcer history. The UBT and fecal antigen are preferred nonendoscopic tests to confirm H. pylori eradication but must be delayed at least 4 weeks after the completion of treatment to avoid confusing bacterial suppression with eradication. The term eradication or cure is used when posttreatment tests conducted 4 weeks after the end of treatment do not detect the organism. Quantitative antibody tests are impractical for posttreatment as antibody titers remain elevated for long periods of time. A negative posttreatment antibody test, however, is considered reliable.
Imaging and Endoscopy
The diagnosis of PUD depends on visualizing the ulcer crater by either upper GI radiography or upper endoscopy (see Table 20-5).1 In the past, radiography was the initial diagnostic procedure of choice because of its lower cost, greater availability, and greater safety. Today, upper endoscopy has replaced radiography because it provides a more accurate diagnosis and permits direct visualization of the ulcer.
CLINICAL COURSE AND PROGNOSIS
The natural history of PUD is characterized by periods of exacerbations and remissions.1 Ulcer pain is usually recognizable and episodic, but symptoms are variable, especially in older adults and for patients taking NSAIDs. Antiulcer medications, including the histamine-2 receptor antagonists (H2RAs), PPIs, and sucralfate, relieve symptoms, accelerate ulcer healing, and reduce the risk of ulcer recurrence, but they do not cure the disease. Both duodenal and gastric ulcers recur unless the underlying cause (H. pylori or NSAID) is removed. Successful H. pylori eradication markedly decreases ulcer recurrence and complications. Prophylactic cotherapy or a COX-2 inhibitor decreases the risk of upper GI events for patients who are taking NSAIDs. GI bleeding, perforation, and obstruction remain troublesome complications of chronic PUD. Mortality for patients with gastric ulcer is slightly higher than in duodenal ulcer and the general population. The development of gastric cancer in H. pylori–infected individuals is a slow process that occurs over 20 to 40 years and is associated with a lifetime risk of less than 1%.2,8
TREATMENT
Desired Outcome
The treatment of chronic PUD varies depending on the etiology of the ulcer (H. pylori or NSAID), whether the ulcer is initial or recurrent, and whether complications have occurred (Fig. 20-5). Overall treatment is aimed at relieving ulcer pain, healing the ulcer, preventing ulcer recurrence, and reducing ulcer-related complications. The goal of therapy for H. pylori–positive patients with an active ulcer, a previously documented ulcer, or a history of an ulcer-related complication is to eradicate H. pylori, heal the ulcer, and cure the disease. Successful eradication heals ulcers and reduces the risk of recurrence for most patients. The goal of therapy for a patient with an NSAID-induced ulcer is to heal the ulcer as rapidly as possible. Patients who are at high risk of developing NSAID ulcers should receive prophylactic cotherapy or be switched to a selective COX-2 inhibitor NSAID (if available) to reduce ulcer risk and related complications. When possible, the most cost-effective drug regimen should be used.
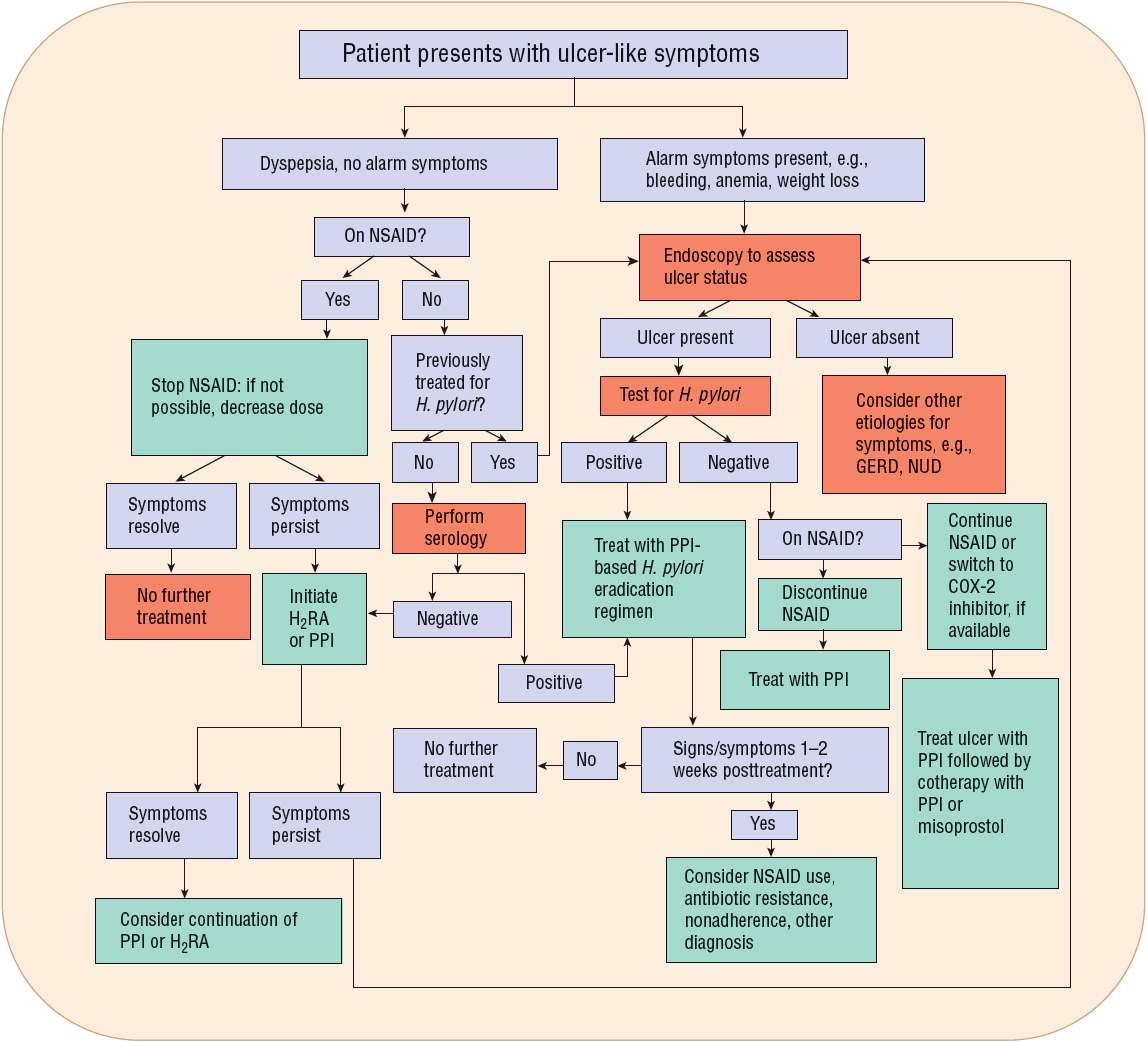
FIGURE 20-5 Algorithm. Guidelines for the evaluation and management of a patient who presents with dyspeptic or ulcer-like symptoms. (COX-2, cyclooxygenase-2; GERD, gastroesophageal reflux disease; H2RA, H2-receptor antagonist; NSAID, nonsteroidal antiinflammatory drug; NUD, nonulcer dyspepsia; PPI, proton pump inhibitor.)
General Approach to Treatment
Antimicrobials such as clarithromycin, metronidazole, amoxicillin, bismuth salts, and antisecretory drugs (PPIs or H2RAs) relieve ulcer symptoms, heal the ulcer, and eradicate H. pylori infection. PPIs are preferred to H2RAs or sucralfate for healing H. pylori–negative NSAID-induced ulcers because they accelerate ulcer healing and provide more effective relief of symptoms. Treatment with a PPI should be extended from 4 to 8-12 weeks if the NSAID must be continued. A PPI-based H. pylori eradication regimen is recommended when the patient with an active ulcer is taking an NSAID and is H. pylori positive. Prophylactic cotherapy with either a PPI or misoprostol decreases ulcer risk and upper GI complications for patients taking nonselective NSAIDs. Selective COX-2 inhibitor NSAIDs (if available) may be used as an alternative to a nonselective NSAID, but their beneficial GI effect when taken with low-dose aspirin is negated and their association with adverse cardiovascular effects reduces their usefulness.
Dietary modifications are important for patients who are unable to tolerate certain foods and beverages. Lifestyle modifications such as reducing stress and stopping cigarette smoking are encouraged. Surgery may be necessary for patients with ulcer-related complications.
Nonpharmacologic Therapy
![]() Patients with PUD should eliminate or reduce psychological stress, cigarette smoking, and the use of NSAIDs (including aspirin). Although there is no “antiulcer diet,” the patient should avoid foods and beverages (e.g., spicy foods, caffeine, and alcohol) that cause dyspepsia or that exacerbate ulcer symptoms. If possible, alternative agents such as acetaminophen or nonacetylated salicylate (e.g., salsalate) should be used for relief of pain. Elective surgery for PUD is rarely performed today because of highly effective medical management. A subset of patients, however, may require emergency surgery for bleeding, perforation, or obstruction. In the past, surgical procedures were performed for medical treatment failures and included vagotomy with pyloroplasty or vagotomy with antrectomy.23 Vagotomy (truncal, selective, or parietal cell) inhibits vagal stimulation of gastric acid. A truncal or selective vagotomy frequently results in postoperative gastric dysfunction and requires a pyloroplasty or antrectomy to facilitate gastric drainage. When an antrectomy is performed, the remaining stomach is anastomosed with the duodenum (Billroth I) or with the jejunum (Billroth II). A vagotomy is unnecessary when an antrectomy is performed for gastric ulcer. Postoperative consequences include postvagotomy diarrhea, dumping syndrome, anemia, and recurrent ulceration.
Patients with PUD should eliminate or reduce psychological stress, cigarette smoking, and the use of NSAIDs (including aspirin). Although there is no “antiulcer diet,” the patient should avoid foods and beverages (e.g., spicy foods, caffeine, and alcohol) that cause dyspepsia or that exacerbate ulcer symptoms. If possible, alternative agents such as acetaminophen or nonacetylated salicylate (e.g., salsalate) should be used for relief of pain. Elective surgery for PUD is rarely performed today because of highly effective medical management. A subset of patients, however, may require emergency surgery for bleeding, perforation, or obstruction. In the past, surgical procedures were performed for medical treatment failures and included vagotomy with pyloroplasty or vagotomy with antrectomy.23 Vagotomy (truncal, selective, or parietal cell) inhibits vagal stimulation of gastric acid. A truncal or selective vagotomy frequently results in postoperative gastric dysfunction and requires a pyloroplasty or antrectomy to facilitate gastric drainage. When an antrectomy is performed, the remaining stomach is anastomosed with the duodenum (Billroth I) or with the jejunum (Billroth II). A vagotomy is unnecessary when an antrectomy is performed for gastric ulcer. Postoperative consequences include postvagotomy diarrhea, dumping syndrome, anemia, and recurrent ulceration.
Pharmacologic Therapy
Recommendations
![]() Table 20-7 presents guidelines for the eradication of infection in H. pylori–positive individuals. Table 20-8 lists regimens used to eradicate H. pylori infection.
Table 20-7 presents guidelines for the eradication of infection in H. pylori–positive individuals. Table 20-8 lists regimens used to eradicate H. pylori infection.
TABLE 20-7 Guidelines for the Eradication of Helicobacter pylori Infection

TABLE 20-8 Drug Regimens Used to Eradicate Helicobacter pylori
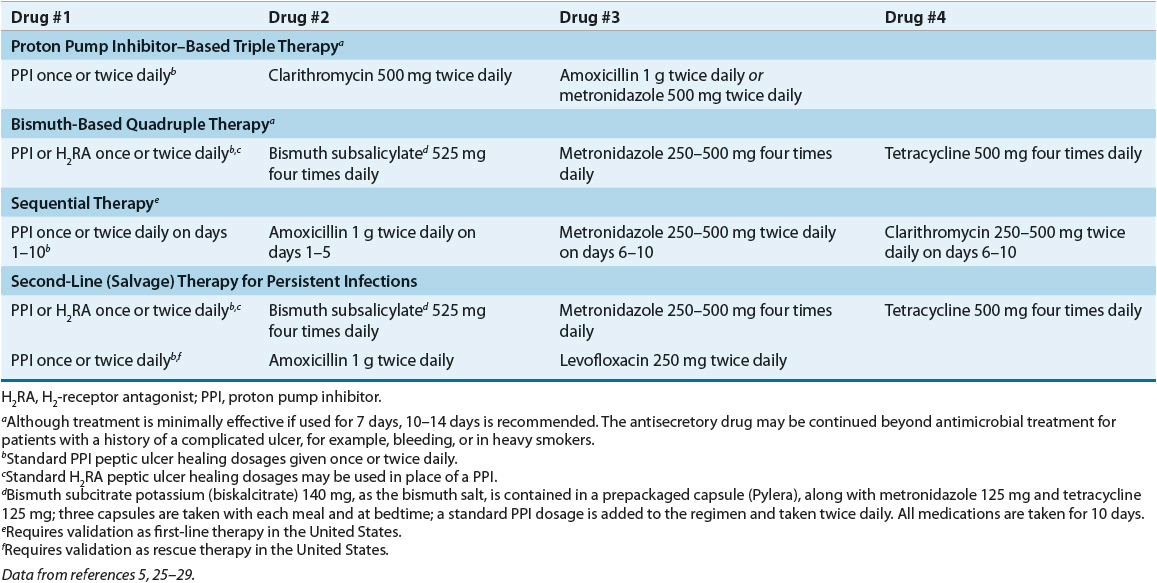
![]() First-line therapy is usually initiated with a PPI-based three-drug regimen for 10 to 14 days. If a second course of treatment is required, the PPI-based three-drug regimen should contain different antibiotics or a four-drug regimen with a bismuth salt, metronidazole, tetracycline, and a PPI should be used.
First-line therapy is usually initiated with a PPI-based three-drug regimen for 10 to 14 days. If a second course of treatment is required, the PPI-based three-drug regimen should contain different antibiotics or a four-drug regimen with a bismuth salt, metronidazole, tetracycline, and a PPI should be used.
Patients with NSAID-induced ulcers should be tested to determine their H. pylori status. If H. pylori positive, treatment should be initiated with a PPI-based three-drug regimen. If H. pylori negative, the NSAID should be discontinued, and the patient treated with a PPI, H2RA, or sucralfate (see Table 20-9). If the NSAID is continued, treatment should be initiated with a PPI (if H. pylori negative) or with a PPI-based three-drug regimen (if H. pylori positive). Cotherapy with a PPI or misoprostol or switching to a selective COX-2 inhibitor (if available) is recommended for patients at risk of developing an ulcer-related complication.
TABLE 20-9 Drug Dosing Table
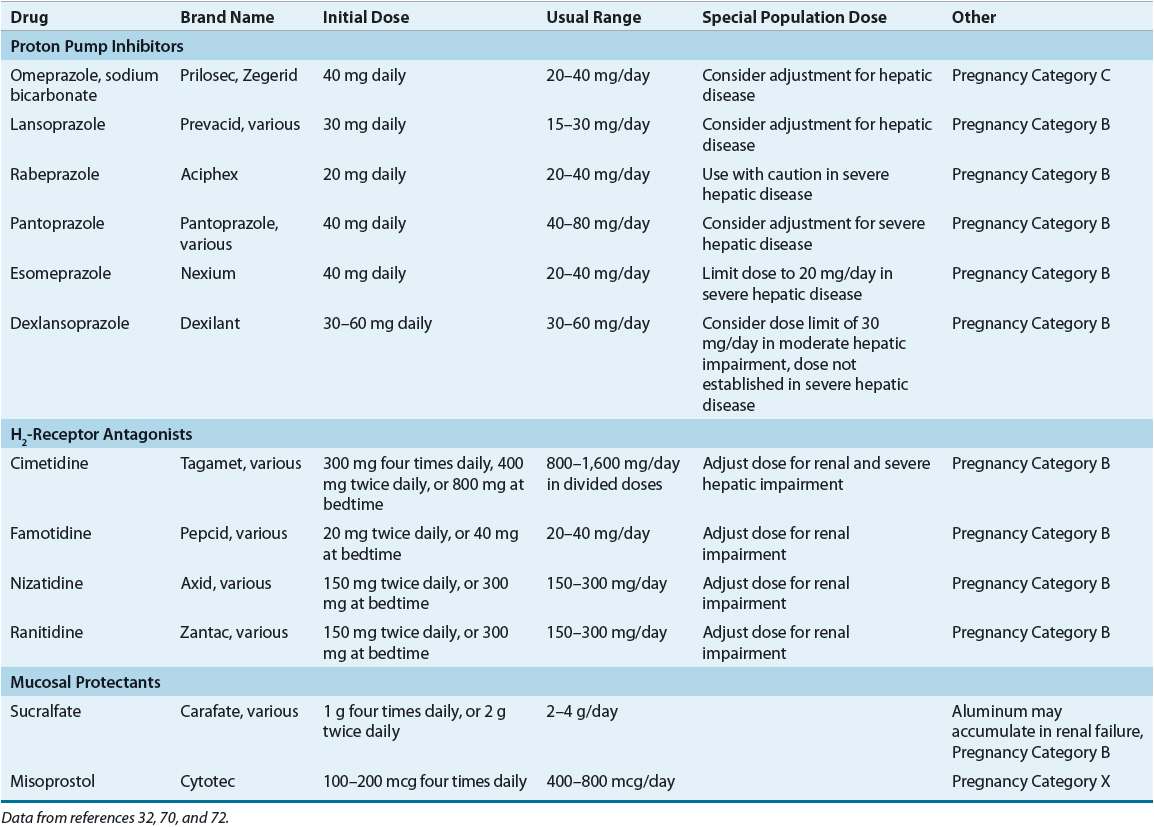
Maintenance therapy with a PPI or H2RA should be limited to high-risk patients with ulcer complications, patients who fail eradication, and those with H. pylori–negative ulcers. Treatment failure is associated with poor medication adherence, antimicrobial resistance, NSAID use, cigarette smoking, acid hypersecretion, or tolerance to the antisecretory effects of an H2RA.
Treatment of H. pylori–Positive Ulcers
This chapter focuses on the eradication of H. pylori in adults.25–29 A discussion of the treatment of H. pylori infection in children is found elsewhere.30,31
The treatment of H. pylori–positive PUD should be effective, well tolerated, easy to adhere to, and cost-effective. Historically, none of these factors have been addressed in a systematic way making it difficult to identify the best evidence-based treatment regimens.1 Successful eradication depends on the drug regimen, resistance to the antibiotics used, duration of therapy, medication adherence, and genetic polymorphism.25–29 H. pylori regimens should have eradication (cure) rates of at least 80% based on intention-to-treat analysis or at least 90% based on per-protocol analysis, and they should minimize the potential for antimicrobial resistance.1,25,29 Not one antibiotic, bismuth salt, or antiulcer drug achieves this goal, but clarithromycin is the single most effective antibiotic. Two-drug regimens that combine a PPI and either amoxicillin or clarithromycin have yielded marginal and variable eradication rates in the United States and are not recommended.1,5 In addition, the use of only one antibiotic is associated with a higher rate of antimicrobial resistance.
Drug regimens (see Table 20-8) that combine an antisecretory drug with two antibiotics (triple therapy) or with two antibiotics and a bismuth salt (quadruple therapy) usually increase eradication rates to acceptable levels and reduce the risk of antimicrobial resistance.5,25–29 When selecting an initial eradication regimen, an antibiotic combination should be used that permits second-line treatment (if necessary) with different antibiotics. The antibiotics that have been most extensively studied and found to be effective in various combinations include clarithromycin, amoxicillin, metronidazole, and tetracycline.1 Because of insufficient data, ampicillin should not be substituted for amoxicillin, doxycycline should not be substituted for tetracycline, and azithromycin or erythromycin should not be substituted for clarithromycin. Antisecretory drugs enhance antibiotic activity and stability by increasing intragastric pH and by decreasing intragastric volume thereby enhancing the topical antibiotic concentration.27
Proton Pump Inhibitor–Based Three-Drug Regimens
PPI-based triple therapy (see Table 20-8) is the initial treatment of choice for eradicating H. pylori (see Table 20-7).5,25–29 The regimens that combine either clarithromycin and amoxicillin or clarithromycin and metronidazole are more effective than the amoxicillin–metronidazole regimen. In most cases, increasing the antibiotic dosage does not improve eradication rates. The clarithromycin–amoxicillin regimen is preferred initially (see Table 20-7), but metronidazole should be substituted for amoxicillin for penicillin-allergic patients unless alcohol is consumed.5,25–27 Unfortunately, eradication rates for PPI-based triple therapy have declined substantially in recent years in North America and Europe due primarily to an increase in clarithromycin-resistant H. pylori strains (see Factors that Predict H. pylori Eradication Outcomes below).5.25–27 Other antibiotics and antibiotic combinations have been investigated, but these regimens should not be used as initial treatment in the United States until well-designed trials confirm their effectiveness.5,27
The recommended duration of therapy in the United States is 10 to 14 days, but the 14-day regimen is preferred in light of the decreasing eradication rate with the PPI-based triple-therapy regimens containing clarithromycin.5 Although a 7-day course has been approved by the FDA and is used in Europe, the longer treatment periods favor higher eradication rates and are less likely to be associated with antimicrobial resistance.5,25–27
Clinical Controversy…
The PPI is an integral part of the three-drug regimen and should be taken 30 to 60 minutes before a meal along with the two antibiotics (see Table 20-8).5,32 Prolonged PPI treatment beyond 2 weeks after eradication is usually not necessary for ulcer healing. A single daily dose of a PPI may be less effective than a twice-daily dose.33 Substitution of one PPI for another is acceptable and does not enhance or diminish H. pylori eradication.32,34 An H2RA should not be substituted for a PPI, as H2RA is associated with lower eradication rates.35,36 Pretreatment with a PPI does not influence H. pylori eradication.37
Bismuth-Based Four-Drug Regimens
Bismuth-based quadruple therapy (see Table 20-8) is recommended as an alternative first-line eradication therapy (see Table 20-7) for those allergic to penicillin.5,25–29 Although this regimen may be used initially, it is often reserved as a second-line therapy after treatment failure with the PPI-based clarithromycin–amoxicillin regimen (see Eradication of H. pylori After Initial Treatment Failure below). Eradication rates for bismuth-based quadruple therapy (bismuth salicylate, metronidazole, tetracycline, and either a PPI or H2RA) are similar to those achieved with PPI-based triple therapy.5,27,38 Eradication rates are comparable when bismuth subcitrate potassium (biskalcitrate) is substituted for bismuth subsalicylate (see Table 20-8).39 Substitution of clarithromycin 250 to 500 mg four times a day for tetracycline yields similar results but increases adverse effects. Bismuth salts have a topical antimicrobial effect.1 The antisecretory drug hastens ulcer healing and relieves pain in patients with an active ulcer. All medications except the PPI should be taken with meals and at bedtime.
The original bismuth-based regimens contained an H2RA in place of a PPI, but a meta-analysis indicated that quadruple therapy with a PPI provides greater efficacy and permits a shorter treatment duration (7 days) when compared with the H2RA-based regimens (10 to 14 days).40 However, a 10- to 14-day duration is recommended in the United States as it generally provides higher eradication rates.5 When treating an active ulcer, the antisecretory drug is usually continued for 2 (PPI) to 4 (H2RA) weeks after stopping bismuth and antibiotics.
Bismuth-based quadruple therapy is the treatment of choice when medication costs are of overriding importance. However, major concerns include a four-times-a-day dosing regimen (see Table 20-8), poor medication adherence, and frequent adverse effects. Although minor adverse effects are more common, the frequency of moderate or severe adverse effects is similar to those reported for the PPI-based triple therapy.41
Sequential Therapy Sequential therapy is a new form of eradication therapy whereby the antibiotics are administered in a sequence rather than all together.5,26,29 The rationale for sequential therapy is to initially treat with antibiotics that rarely promote resistance (e.g., amoxicillin) to reduce the bacterial load and preexisting resistant organisms and then to follow with different antibiotics (e.g., clarithromycin and metronidazole) to kill the remaining organisms.1 Treatment consists of a PPI and amoxicillin for 5 days followed by a PPI, clarithromycin, and tinidazole (or metronidazole) for an additional 5 days (see Table 20-8).5,26,42 Although this regimen has achieved eradication rates that are superior to the PPI-based three-drug regimens containing clarithromycin,42 the regimen requires a change in medication midtreatment, which may contribute to nonadherence.43 The advantages of sequential therapy need to be confirmed in the United States before it can be recommended as a first-line H. pylori eradication therapy (see Table 20-7).5,26,27
Eradication of H. pylori After Initial Treatment Failure
H. pylori eradication is often more difficult after initial treatment fails and successful eradication after retreatment is extremely variable.5,44 Treatment failures should be referred to a gastroenterologist for further diagnostic evaluation. Second-line (salvage) treatment should (a) use antibiotics that were not previously used during initial therapy; (b) use antibiotics that are not associated with resistance; (c) use a drug that has a topical effect such as bismuth; and (d) extend the duration of treatment to 14 days.5,44,45 The most commonly used second-line therapy, after unsuccessful initial treatment with a PPI–amoxicillin–clarithromycin regimen, is a 14-day course of the PPI-based quadruple therapy (see Table 20-8).5,26,27,45 A levofloxacin-containing regimen (see Table 20-8) may be an alternative second-line eradication regimen and may be better tolerated than PPI-based quadruple therapy (see Table 20-7).46 Additionally, the levofloxacin regimen may serve as an alternative to PPI–clarithromycin–metronidazole usually recommended in penicillin-allergic patients.47 However, concerns about using fluoroquinolones for H. pylori eradication are related to the development of resistance and adverse effects such as tendonitis and hepatotoxicity.26 Other second-line regimens that include rifabutin and furazolidone are discussed elsewhere.5,25–27
Factors that Predict H. pylori Eradication Outcomes
Factors that predict H. pylori eradication outcomes include antibiotic resistance, poor medication adherence, short duration of therapy, CagA status, high bacterial load, low intragastric pH, and genetic polymorphism.5,45,48–50 Medication adherence decreases with multiple medications, increased frequency of administration, intolerable adverse effects, and costly drug regimens. One meta-analysis reported that CYP2C19 polymorphism may alter the effect of PPIs on gastric acid secretion thereby influencing eradication outcomes.49 Tolerability varies with different regimens.1,5 Common adverse effects include nausea, vomiting, abdominal pain, diarrhea, and taste disturbances (metronidazole and clarithromycin). Adverse effects with metronidazole are dose related (especially when >1 g/day) and include a disulfiram-like reaction with alcohol. Tetracycline may cause photosensitivity and should not be used in children because of possible tooth discoloration. Bismuth salts may cause darkening of the stool and tongue. Antibiotic-associated diarrhea and Clostridium difficile–associated disease can occur. Oral thrush and vaginal candidiasis may also occur.
An important predictor of H. pylori eradication is the presence or absence of resistant microorganisms.5,50–52 U.S. data from 1993 to 1999 report resistance rates among H. pylori strains for metronidazole (37%), clarithromycin (10%), and amoxicillin (1.4%).51 Data from 1998 to 2002 reveal rates of 25% for metronidazole, 13% for clarithromycin, and 0.9% for amoxicillin.52 In one study, the proportion of clarithromycin resistance increased with increasing courses of macrolide antibiotics, from 7% resistance with no prior exposure to 80% resistance with ≥5 courses of macrolides. It is possible that the increased rate of clarithromycin resistance partially explains the decrease in efficacy of clarithromycin-containing regimens. The clinical importance of metronidazole resistance remains uncertain, as resistance can be overcome by using higher dosages and combining metronidazole with other antibiotics.5 Resistance to tetracycline and amoxicillin is uncommon.5 Resistance to bismuth has not been reported. The role of antibiotic sensitivity testing prior to initiating H. pylori treatment has not been established.
Probiotics
Probiotics (e.g., strains of Lactobacillus and Bifidobacterium) and foodstuffs (e.g., cranberry juice and some milk proteins) with bioactive components have been used proactively to control H. pylori colonization in at-risk individuals and, when taken as a supplement to eradication therapy, may have a role in improving H. pylori eradication and reducing the adverse effects of PPI-based triple therapy.53–55 However, the administration of probiotics alone does not eradicate H. pylori infection. In the future, the regular intake of probiotics may constitute a low-cost alternative for individuals who are at risk for H. pylori infection and, in combination with antibiotics, augment eradication rates. These preliminary data are encouraging and warrant more research in this area.
Treatment of NSAID-Induced Ulcers
Nonselective NSAIDs should be discontinued (when possible) on confirmation of an active ulcer. If the NSAID is stopped, most uncomplicated ulcers heal with standard regimens of an H2RA, PPI, or sucralfate (see Table 20-9).1,13,29 However, PPIs are usually preferred because they provide more rapid symptom relief and ulcer healing. If the NSAID is continued despite ulceration, consideration should be given to reducing the NSAID dose, switching to acetaminophen or a nonacetylated salicylate, or using a more selective COX-2 inhibitor (see Table 20-3). PPIs are the drugs of choice when the NSAID is continued, as potent acid suppression is required to accelerate ulcer healing.1,13,29 If the ulcer is H. pylori positive, eradication should be initiated with a regimen that contains a PPI.1,13,29
Strategies to Reduce the Risk of NSAID Ulcer and GI Complications There are three therapeutic approaches to reducing the risk of NSAID ulcers and related upper GI complications (see Table 20-10). Medical cotherapy with either a PPI or misoprostol decreases ulcer risk and GI complications in high-risk patients.12–14,16,19,29 The use of a selective COX-2 inhibitor instead of a nonselective NSAID also decreases risk of ulcers and upper GI events.12–14,16,18,19,29 Unfortunately, these strategies do not completely eliminate ulcers and complications for patients at the “highest risk.” When selecting a gastroprotective strategy, the GI benefits must be balanced against the cardiovascular risks associated with selective COX-2 inhibitor NSAIDs, nonselective NSAIDs, and concomitant antiplatelet therapy.12–16 Strategies aimed at reducing the topical irritant effects of nonselective NSAIDs, for example, prodrugs, slow-release formulations, and enteric-coated products, do not prevent ulcers or GI complications.
TABLE 20-10 Drug Monitoring Table