http://evolve.elsevier.com/Edmunds/NP/
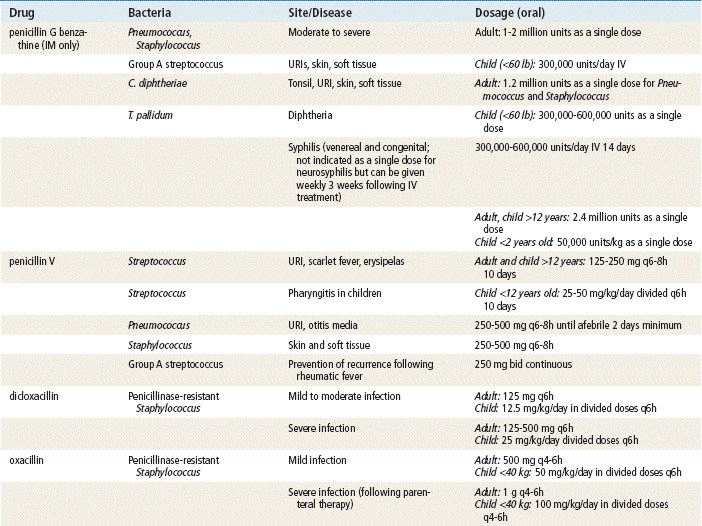
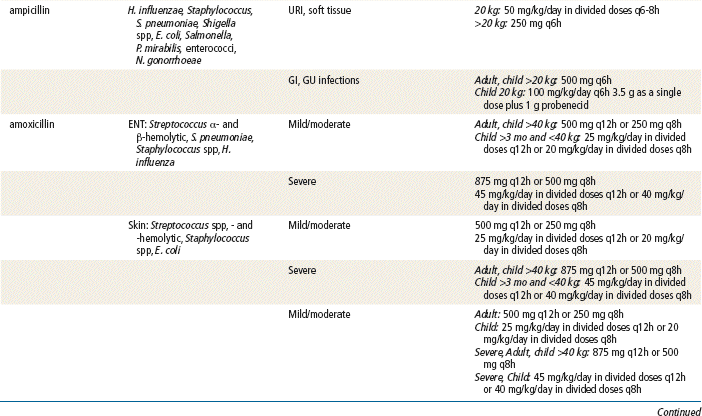
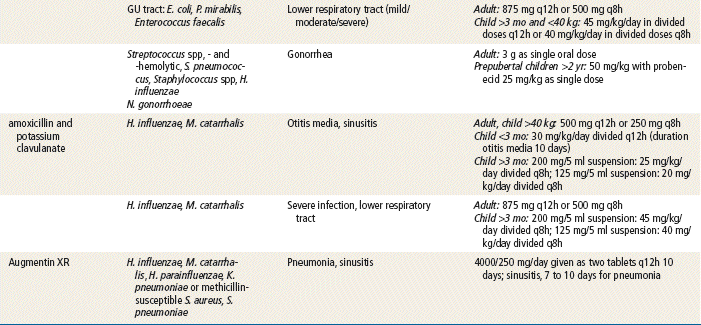
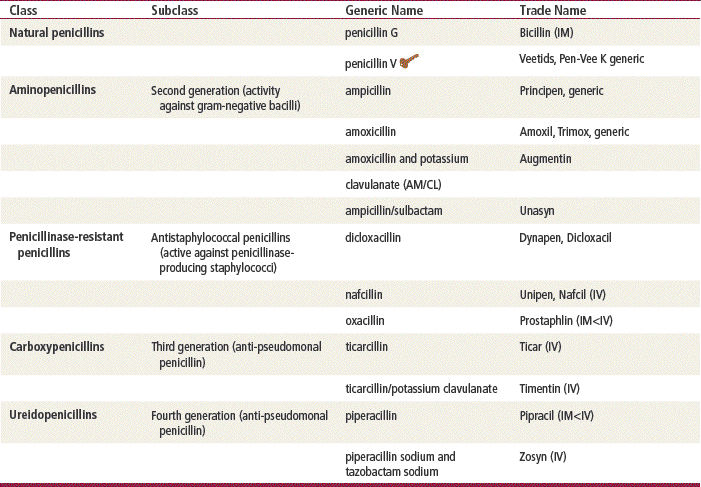
Sir Alexander Fleming, a British scientist, discovered penicillin in 1928, and the medicine has been available since the 1940s. Since that time, penicillins have remained an extremely important class of antibiotics for treating most gram-positive bacteria; some penicillins can treat gram-negative bacteria. Penicillins are considered the safest antibiotics. Important penicillins for the primary care provider include penicillin VK, amoxicillin, amoxicillin/clavulanate, and dicloxacillin. These generally are indicated in the treatment of mild to moderately severe infections caused by penicillin-sensitive microorganisms. They are used extensively for empirical therapy and for treatment determined by culture. Different penicillins exhibit different antimicrobial activity. This chapter focuses on the outpatient use of oral penicillins. Nafcillin is available as IV treatment only and is not discussed further. The carboxypenicillins and the ureidopenicillins are active against a large number of infectious diseases. These generally are given IV in a hospital setting and are not discussed further. Other β-lactam drugs, the carbapenems (e.g., ertapenem, imipenem, and meropenem), are similar in chemical structure to the penicillins. These drugs are used in hospitals and are given IM or IV for severe infections; these also will not be discussed further.
Therapeutic Overview
Before prescribing any penicillin, check for drug allergy. The incidence of penicillin allergy is unknown but is probably between 5% and 10%. Life-threatening anaphylaxis is estimated at 0.01% to 0.05%. A patient’s report of penicillin allergy correlates poorly with a positive skin test. A patient with a negative skin test has a very low probability of an allergic reaction to penicillin. Among patients with a positive skin test for penicillin, less than 5% will have an immediate reaction to a cephalosporin. Current recommendations are that if a penicillin skin test is positive, the patient should avoid β-lactam antimicrobials or should be considered for penicillin or cephalosporin desensitization. Cross reactivity to carbapenems is also possible; therefore, a carbapenem may not be a reasonable choice for penicillin-allergic patients.
Resistance
Microbial resistance to the penicillins, now common, has become a major limitation to their use. Resistance of bacteria to penicillins and other β-lactam antibiotics occurs through three mechanisms. The most important mechanism involves bacteria-producing β-lactamase, which breaks down the β-lactam ring and renders the penicillin inactive. Staphylococcus aureus is one of the most important of these organisms. Methicillin-resistant Staphylococcus aureus (MRSA) is now resistant to most antibiotics and is very difficult to treat. Methicillin is no longer marketed in the United States because of resistance and toxicity. Inhibitors of the β-lactamases such as clavulanic acid, sulbactam, and tazobactam often are used in combination with certain penicillins, to prevent their inactivation.
The second mechanism of resistance is diminished permeability of the drug through the bacterial outer cell membrane. The antibiotic is unable to penetrate the cell wall, especially of gram-negative bacteria. The last mechanism involves decreased binding of the drug at its target sites on the inner bacterial membrane.
Mechanism of Action
Penicillin is a derivative of 6-aminopenicillanic acid that is composed of a distinct four-membered β-lactam ring fused to a five-membered thiazolidine ring; this constitutes the chemical structure. Penicillin subclasses have additional chemical constituents that bestow differences in antimicrobial activity, susceptibility to acid, enzyme hydrolysis, and biodisposition. Many biosynthetic types of penicillin have been created through the introduction of diverse acids, amines, or amides into developing penicillin molds, to render products superior to the natural penicillins.
Penicillins, which are bactericidal against susceptible organisms, disrupt synthesis of the bacterial cell wall and compete for and bind to specific enzyme proteins that catalyze transpeptidation and cross-linking. The enzymes to which they bind are called penicillin-binding proteins (PBPs). They consist of transpeptidases, transglycosylases, and D-alanine carboxykinase and are implicated in the final phases of building and reshaping of the bacterial cell wall while it is growing and dividing. This action interferes with the biosynthesis of mucopeptides and prevents linkage of structural components of the cell wall. After the penicillin molecules bind and inhibit the transpeptidase enzymes, susceptible bacteria are no longer able to lay protein cross-links across the peptidoglycan backbone of the cell wall. In addition to being structurally weak, this formation is thought to catalyze the activation of autolytic enzymes in the cell wall that cause progressive bacterial lysis.
Treatment Principles
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Key drug.
Key drug.