Table 37-1. Average CrCl Values (Normal Renal Function)
Age (term) | Value (ml/min/1.73 m2) |
5–7 days | 50.6 ± 5.8 |
1–2 months | 64.6 ± 5.8 |
5–8 months | 87.7 ± 11.9 |
9–12 months | 86.9 ± 8.4 |
> 18 months (male) | 124 ± 26 |
> 18 months (female) | 109 ± 13.5 |
Adult (male) | 105 ± 14 |
Adult (female) | 95 ± 18 |
■ It may be affected by the presence of maternal serum creatinine (SCr) over the first week of life (i.e., false underestimation of CrCl).
■ The Schwartz equation may be used for estimating GFR in pediatric patients:
GFR(mL/min/1.73 m2) = k x (length in cm)/SCr
where k = proportionality constant that changes with age and sex (Table 37-2).
Alternately, the Bedside Chronic Kidney Disease in Children (CKiD) equation, also referred to as the updated or modified Schwartz equation, may be a better estimate of GFR in pediatric patients with moderate to severe kidney disease:
GFR(mL/min/1.73 m2) = 0.413 x (height in cm)/SCr
Other Pediatric Drug Issues
■ Digoxin-like immunoreactive substance (DLIS) is produced in infants. DLIS may interfere with digoxin assays and falsely elevate concentrations.
■ Di(2-ethylhexyl)phthalate (DEHP), a plasticizer contained in intravenous (IV) bags, is shown to have an effect on the male reproductive system. Pediatric patients at highest risk of DEHP exposure are neonates on extracorporeal membrane oxygenation, those receiving parenteral and enteral nutrition, and those receiving plasma exchange transfusions.
■ Propylene glycol, an additive used to promote stability in certain IV medications, can cause hyperosmolarity in infants.
■ Potentially fatal central nervous system and respiratory depression because of morphine accumulation may occur in neonates of mothers who are CYP2D6 ultrarapid metabolizers and are taking codeine for postpartum pain.
Table 37-2. Proportionality Constant for Calculation of CrCl Using the Schwartz Equationa
Age | k |
Low birth weight ≤ 1 year | 0.33 |
Full term ≤ 1 year | 0.45 |
1–12 years | 0.55 |
14–21 years (female) | 0.55 |
14–21 years (male) | 0.70 |
a. Schwartz, Brion, Spitzer, 1987.
37-4. Specific Infections and Disease States in the Pediatric Population
Otitis Media
Otitis media is an inflammatory process of the middle ear.
Classification
■ Acute otitis media is the rapid onset of signs and symptoms of inflammation in the middle ear. Uncomplicated acute otitis media is not accompanied by otorrhea (discharge from the ear).
• Nonsevere: Mild otalgia (ear pain) for < 48 hours and temperature < 39°C or 102.2°F
• Severe: Moderate to severe otalgia, otalgia for at least 48 hours, or fever ≥ 39°C or 102.2°F
■ Recurrent otitis media is the diagnosis of three or more separate episodes of acute otitis media within a 6-month period or four episodes within a year (with at least one episode in the past 6 months).
■ Otitis media with effusion is inflammation of the middle ear with the presence of fluid in the middle ear (effusion) without the associated signs or symptoms of acute infection.
Clinical presentation
Signs and symptoms include fever, otalgia (often manifested as ear tugging or pulling), otorrhea, changes in balance or hearing, irritability, difficulty sleeping, lethargy, anorexia, vomiting, and diarrhea. Associated findings may be runny nose, congestion, or cough.
Pathophysiology
Eustachian tube dysfunction
The infant’s eustachian tube is shorter and more horizontal than that of the adult, thus preventing drainage of middle ear secretions into the nasopharynx and promoting pooling of secretions in the middle ear. Anatomic abnormalities increase risk (e.g., cleft palate, adenoid hypertrophy). An immature immune system or altered host defenses also increase risk, as do viral infections and allergies.
Risk factors include male gender; Native American, Canadian Eskimo, or Alaskan descent; family history of acute otitis media or respiratory tract infection; early age of first episode (earlier age is associated with greater severity and recurrence); day care environment; parental smoking; lack of breast-feeding in infancy; and pacifier use.
Complications include mastoiditis, meningitis, subdural empyema, hearing loss, and delayed speech and language development.
Microbial pathogens
Historically, up to 50% of cases of otitis media were thought to be viral in origin. However, with accurate diagnosis of acute otitis media (i.e., differentiating between acute disease and otitis media with effusion), the majority of cases are bacterial with or without a viral component. The primary bacteria responsible for acute otitis media are discussed below.
■ Streptococcus pneumoniae has been responsible over time for the majority of bacterial otitis media cases. Bacterial resistance, which occurs primarily through alteration in penicillin-binding protein (decreased affinity for binding sites), is common.
■ Haemophilus influenzae (primarily nonencapsulated or nontypeable strains) has been considered the second-most-common organism responsible for bacterial otitis media cases. Bacterial resistance occurs through β-lactamase production.
■ Moraxella catarrhalis is the third-most-common organism responsible for bacterial otitis media cases. Almost all strains are β-lactamase producing.
With the advent of the pneumococcal 7-valent (PCV7) and 13-valent (PCV13) vaccines, the otopathogens responsible for acute otitis media are changing. The incidence of acute otitis media because of S. pneumoniae and H. influenzae strains are now approximately equal. With respect to S. pneumoniae, the majority of strains identified in acute otitis media cases are those that are not represented in the vaccine.
Diagnosis
In 2004, the American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP) published clinical practice guidelines on the diagnosis and management of acute otitis media. These guidelines established diagnostic criteria and the use of pneumatic otoscope or tympanometry to differentiate between episodes of acute otitis media and otitis media with effusion. The guidelines were also the first official support in North America for an observation therapy option in acute otitis media for select patients. In 2013, these guidelines were revised to further strengthen the diagnostic criteria. It is important to note that the 2013 guidelines apply to the diagnosis and management of uncomplicated acute otitis media in infants and children 6 months to 12 years of age. For management in infants less than 6 months of age, the earlier guidelines may still be used.
When middle ear disease is present, otoscopic examination determines color, translucency, and position. Redness or opacity of membrane, absence of light reflection, or bulging membrane will be observed.
Pneumatic otoscopic examination determines mobility of the tympanic membrane (i.e., presence or absence of effusion). The membrane will not move briskly with positive and negative pressure if effusion is present. Tympanometry may also be used to determine the presence of middle ear effusion.
Tympanocentesis (i.e., a needle is inserted through the tympanic membrane to withdraw fluid) allows for culture and identification of the pathogen.
According to the 2013 guidelines, middle ear effusion on examination with pneumatic otoscope or with tympanometry must be present to diagnose acute otitis media. In addition, acute otitis media should be diagnosed with moderate to severe bulging of the tympanic membrane or new onset of otorrhea. Alternately, acute otitis media may be diagnosed with mild bulging of the tympanic membrane and recent onset (< 48 hours) of ear pain or intense erythema of the tympanic membrane.
Treatment principles and goals
■ Assess and control pain.
■ Eradicate infection.
■ Prevent complications.
■ Avoid unnecessary antibiotic therapy.
■ Improve compliance.
■ Eliminate presence of effusion.
■ Prevent recurrence.
Drug therapy
Many episodes of otitis media will have spontaneous resolution; however, because of a risk of complications from untreated otitis media, antimicrobials remain the mainstay of therapy. Observation therapy may be appropriate in select patients.
First-line therapy
High-dose amoxicillin (Amoxil) is the recommended first-line treatment for acute otitis media. Amoxicillin has excellent in vitro activity against S. pneumoniae and most H. influenzae. It has the optimal pharmacodynamic profile of the available agents and reaches good concentrations in middle ear fluid. Amoxicillin has an excellent safety and efficacy profile with a narrow spectrum of activity. It is palatable and inexpensive. It may overcome drug-resistant S. pneumoniae with higher doses (i.e., achieves greater concentrations in middle ear fluid). It does not eradicate β-lactamase–producing organisms.
For penicillin-allergic patients, cefdinir (Omnicef), cefpodoxime (Vantin), cefuroxime (Ceftin), or ceftriaxone (Rocephin) may be used.
Amoxicillin-clavulanate (Augmentin) should be used as first-line therapy in patients who have received amoxicillin in the past 30 days, those with conjunctivitis, or those in whom β-lactamase-positive H. influenzae or M. catarrhalis is indicated.
Dosing issues and drug resistance
Antimicrobial treatment options are described in Table 37-3.
Dosages are as follows:
■ Amoxicillin (Amoxil): A high dose is recommended (80–90 mg/kg/day).
■ Amoxicillin-clavulanate (Augmentin): A high dose is recommended (90 mg/kg/day of amoxicillin with 6.4 mg/kg/day of clavulanate). Maintain daily clavulanate dose < 10 mg/kg/day to prevent diarrhea.
■ Ceftriaxone (Rocephin): An intramuscular (IM) dose of 50 mg/kg/dose (single dose versus three daily doses) is recommended. More than a single dose may be necessary to prevent recurrence within 5–7 days of the initial dose.
Duration of therapy
Two courses of therapy are possible:
■ Standard course (10-days)
■ Shorter course (1–7 days)
Advantages of the shorter course are improved compliance, decreased adverse effects of drug therapy, decreased risk of bacterial resistance, and lower costs. Disadvantages are delayed or no cure, increased risk of complications from untreated acute otitis media, and greater risk of recurrence.
Table 37-3. Antimicrobial Therapy for Acute Otitis Media
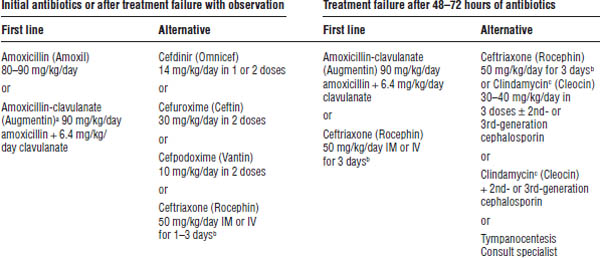
a. Amoxicillin-clavulanate is first-line therapy in patients who have received amoxicillin in the past 30 days, those with conjunctivitis, those with a history of recurrent otitis media unresponsive to amoxicillin, or those in whom β-lactamase activity for H. influenzae or M. catarrhalis is indicated.
b. More than 1 ceftriaxone dose may be needed to prevent recurrence.
c. Clindamycin does not have activity against H. influenzae. It should be used when penicillin-resistant S. pneumoniae is suspected.
The 2013 guidelines recommend the standard 10-day course in children < 2 years of age or severe symptoms at any age. A 7-day course may be used in children 2–5 years of age (and a 5–7 day course in children 6 years and older) with mild to moderate acute otitis media.
Other therapy
Antipyretics (acetaminophen [Tylenol] and ibuprofen [Motrin]) or analgesics may be used. Use acetaminophen with caution in high doses to avoid hepatotoxicity. Use ibuprofen with caution in patients with vomiting, diarrhea, and poor fluid intake, because dehydration predisposes them to ibuprofen-induced renal insufficiency. Avoid alternating antipyretic therapy. Encourage parents to choose one agent, inform them of any adverse effects, and educate them about symptoms of these effects (e.g., hepatotoxicity or renal insufficiency).
Narcotic analgesics may be used for moderate to severe pain not controlled with acetaminophen or ibuprofen.
Topical analgesics include otic solutions, such as antipyrine-benzocaine (Auralgan, Americaine Otic), and naturopathic otic solutions.
Topical antimicrobials may have a place in therapy, particularly with ruptured tympanic membranes (fluoroquinolone or fluoroquinolone and steroid combination otic suspensions [Floxin, Cipro HC, Ciprodex]).
Antihistamines and decongestants are ineffective at eliminating effusion or relieving symptoms. Use them only if indicated for other signs or symptoms.
Patient instructions and counseling
■ Complete the entire course of prescribed antibiotics.
■ Shake bottle well before administering dose. Follow labeling regarding temperature for storage of medication.
■ Contact the health care provider if patient develops a rash or has difficulty breathing, or if symptoms persist after 72 hours of initiating therapy.
Adverse drug events
■ Gastrointestinal effects: Nausea and diarrhea; discoloration of stools (with cefdinir [Omnicef])
■ Hypersensitivity: Rash, anaphylaxis
Nondrug therapy
Local heat or cold therapy may be used (counsel the caregiver on appropriate use and technique to prevent burn injury).
Tympanostomy tubes decrease recurrent episodes, restore hearing, and relieve discomfort. Risks include anesthesia and permanent tympanic membrane scarring.
Observation therapy
Observation therapy is appropriate only when follow-up at 48–72 hours can be ensured and antimicrobials initiated if symptoms persist or worsen. This therapy is not appropriate for the following patients:
■ Infants < 6 months of age
■ Infants and children between 6 months and 2 years of age with otorrhea, severe symptoms (toxic-appearing child, otalgia > 48 hours, or temperature ≥39°C or 102.2°F), or bilateral disease
■ Children ≥ 2 years of age with otorrhea or severe symptoms as described above
Immunization and immunoprophylaxis
Pneumococcal conjugate vaccination should provide some protection against strains responsible for a majority of bacterial otitis media.
H. influenzae type B vaccination is of no benefit in otitis media. Most strains causing otitis media are nontypeable and not prevented by vaccination.
Killed and live-attenuated intranasal influenza vaccine may decrease episodes of acute otitis media during the respiratory illness season. Annual influenza vaccination is recommended for infants and children age 6 months and older as part of the childhood immunization schedule.
Risk reduction
■ Encourage exclusive breast-feeding for 6 months.
■ Eliminate passive exposure to tobacco smoke.
■ Avoid supine bottle feeding.
■ Reduce or eliminate pacifier use after 6 months of age.
■ Reduce incidence of upper respiratory infections by altering day care attendance (when possible).
Recurrent otitis media
The use of antimicrobials for otitis media prophylaxis is not recommended. Risk factor reduction in this group of patients is important. Tympanostomy tubes may be recommended with recurrent otitis media.
Otitis media with effusion
In 2004, the AAP, AAFP, and the American Academy of Otolaryngology–Head and Neck Surgery published a clinical practice guideline on otitis media with effusion. It applies to infants and children (2 months to 12 years of age) with or without developmental disabilities or underlying conditions that predispose patients to otitis media with effusion. Recommendations include the following:
■ Use pneumatic otoscopy as the primary diagnostic method.
■ Distinguish otitis media with effusion from acute otitis media.
■ Determine the risk of speech, language, and learning problems.
• At-risk children: More rapid evaluation and intervention
• Children not at risk: Watchful waiting for 3 months from date of onset or diagnosis
■ No role exists for antihistamines, decongestants, antimicrobials, or corticosteroids.
■ Hearing testing is recommended with effusion lasting ≥ 3 months or when language delay, learning problems, or hearing loss occur.
■ For persistent otitis media with effusion (not at risk), perform evaluations every 3–6 months until effusion is resolved, hearing loss is identified, structural abnormalities are suspected, or the child becomes a surgical candidate (tympanostomy tube insertion is preferred).
Otitis Externa
Otitis externa is an inflammation of the outer ear canal, also referred to as swimmer’s ear.
Clinical presentation
Patients present with itching, pain, otic exudate, and hearing impairment.
Pathophysiology
Moisture is present in the ear canal, and the integrity of the ear canal is disrupted. The most common organisms are Pseudomonas aeruginosa and Staphylococcus aureus. Other pathogens include fungi and Bacillus and Proteus species.
Therapy consists of antibiotic or steroid otic preparations such as neomycin, polymyxin B, and hydrocortisone (Cortisporin Otic), or neomycin, colistin, and hydrocortisone (Coly-Mycin S Otic). Fluoroquinolone otic preparations such as ciprofloxacin (Cipro HC) and ofloxacin (Floxin) can also be used, as well as acetic acid and hydrocortisone otic preparations (VoSol HC Otic) or oral analgesics.
Preventive measures include drying ears after exposure to moisture; using drops containing isopropyl alcohol, with or without acetic acid to reduce pH; and avoiding cotton swabs.
Otic drops should be administered to a pediatric patient as follows:
- Wash hands before and after administration.
- Warm otic drops to room temperature by holding bottle in hands for several minutes. Avoid instilling cold or hot drops into the ear canal.
- Shake the bottle if indicated on the label.
- Tilt the child’s head to the side, or have the child lie down.
- Pull the child’s ear backward and upward (for children < 3 years, pull ear backward and downward), and instill the drops in the ear canal. Do not put the dropper bottle inside the ear canal. To remain free from contamination, it should not come into contact with the ear.
- Press gently on the small flap over the ear to push the drops into the canal.
- Have the child remain in the same position for the period of time indicated in the labeling. If this is not possible, place a cotton ball gently into the ear to prevent the drops from draining out of the ear canal.
- Wipe excess medication from the outside of the ear.
Cystic Fibrosis
Cystic fibrosis is an autosomal recessive disease of exocrine gland function resulting in abnormal mucus production.
Genetic classification
Cystic fibrosis is the result of a gene mutation on the long arm of chromosome 7. The protein encoded by this gene, the cystic fibrosis transmembrane conductance regulator (CFTR), is a channel involved in the transport of water and electrolytes.
Defects in processing
The most common genetic mutation involves a 3-base-pair deletion at position 508 (ΔF508). Patients homozygous for ΔF508 are pancreatic insufficient.
Prognosis is not as good as that for patients who are pancreatic sufficient. Defects exist in protein production, regulation, and conduction.
Clinical presentation
Pulmonary complications
Initial manifestations include chronic cough, wheezing, hyperinflation of lungs, or lower respiratory tract infections. Patients present with hypoxia, clubbing, labored breathing, and acute respiratory exacerbations (fever, sputum production, increased oxygen requirements, dyspnea); changes in forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and residual volume; and the development of a chronic obstructive picture as the disease progresses.
Gastrointestinal complications
Gastrointestinal complications include poor digestion of proteins and fats, resulting in foul-smelling steatorrhea, and distal intestinal obstruction (commonly manifested as vomiting of bilious material, abdominal distension, and pain).
Infants may have meconium ileus and gastroesophageal reflux.
Other complications
Patients may also have the following complications:
■ Cirrhosis, cholelithiasis
■ Problems with pancreatic function
■ Insulin insufficiency, diabetes mellitus
■ Malnutrition
■ Nasal polyps and sinusitis, anemia, arthritis, osteopenia, osteoporosis
Pathophysiology
A defect exists in the chloride transport channel in secretory epithelial cells. Normally, chloride is transported out of blood followed by sodium and water. However, with cystic fibrosis, decreased chloride and water secretion and increased sodium absorption lead to thick, dehydrated secretions and mucus. Exocrine gland involvement includes pancreas, hepatobiliary ducts, gastrointestinal tract, and the lungs (secretions build up and block airways and pancreatic and hepatobiliary exocrine flow).
Pulmonary system
Initial obstruction of small airways with mucus plugging results in bronchiolitis and persistence of bacteria, as follows:
■ Early bacterial pathogens: S. aureus is the primary pathogen in younger patients.
■ Later bacterial pathogens: P. aeruginosa is the primary pathogen in late childhood.
■ Other bacterial pathogens: Alcaligenes, Stenotrophomonas, Mycobacteria, Aspergillus, and Burkholderia can be present.
Viral pathogens can also be present.
Chronic pulmonary infection and inflammation progress to large airway and eventual chronic obstructive disease.
Pancreatic system
Pancreatic enzyme insufficiency (trypsin, chymotrypsin, lipases, amylase) and decreased bicarbonate secretion (necessary for optimal pancreatic enzyme activity) can occur. Thus, maldigestion of fats and proteins and fat-soluble vitamin deficiency may develop.
Insulin insufficiency (resistance and decreased secretion) leads to glucose intolerance and the development of diabetes mellitus (occurs later in the disease process and may be associated with increased morbidity and mortality).
Biliary system
Biliary cirrhosis or fatty infiltration may lead to portal hypertension, development of bleeding varices, hypersplenism, and cholelithiasis.
Sweat glands
A high concentration of sodium and chloride exists in sweat (representing the failure of sweat glands to reabsorb sodium and chloride).
Reproductive system
Male infertility is common because of bilateral absence of vas deferens. Female infertility is due to abnormal cervical mucus.
Diagnostic criteria
Testing for cystic fibrosis is required in all states as part of the newborn screen. Laboratory confirmation of CFTR dysfunction should be obtained through sweat chloride analysis (i.e., administration of pilocarpine):
■ Sweat is collected, and electrolytes are measured.
■ Chloride of 60 mEq/L or more is diagnostic.
■ Chloride of 40–59 mEq/L is indeterminate, and tests may need to be repeated.
Treatment goals
■ Halt or decrease disease progression.
■ Maintain normal growth and development and nutrition status.
■ Maintain pulmonary function.
■ Optimize drug therapy for pharmacokinetic differences in cystic fibrosis patients.
Drug therapy
Drug therapy for cystic fibrosis is described in Table 37-4.
Pancreatic enzyme replacement products have historically been marketed in the United States as unapproved products. Because of variability in the amount of enzymes in these products, the U.S. Food and Drug Administration (FDA) mandated, effective April 2010, that all manufacturers of pancreatic enzyme replacement products submit a new drug application with the FDA to continue to market their products in the United States. As a result of this mandate, many existing products have been removed from the market. As of May 2014, only six FDA-approved pancreatic enzyme replacement products are available in the United States (see Table 37-4).
Antibiotic therapy in acute exacerbations
Empiric therapy should be used initially. The patient should then be treated on the basis of sputum culture and sensitivity. Administer intravenously two antibiotics for 14–21 days in combination with aggressive therapy for clearance of secretions. Provide coverage for S. aureus and P. aeruginosa.
Double coverage of antibiotics is recommended when Pseudomonas species are suspected, so typically use an antipseudomonal penicillin (piperacillin [Pipracil], mezlocillin [Mezlin], piperacillin-tazobactam [Zosyn], ticarcillin-clavulanate [Timentin], ticarcillin [Ticar], aztreonam [Azactam]) or cephalosporin (ceftazidime [Fortaz]), a carbapenem (meropenem [Merrem], imipenem-cilastatin [Primaxin]), or a quinolone (ciprofloxacin [Cipro]) plus an aminoglycoside (tobramycin [Nebcin]).
Most S. aureus are β-lactamase producers, so use an extended spectrum penicillin–β-lactamase inhibitor combination (e.g., ticarcillin-clavulanate [Timentin]). Use vancomycin (Vancocin) for methicillin-resistant S. aureus.
Table 37-4. Drug Therapy for Cystic Fibrosis
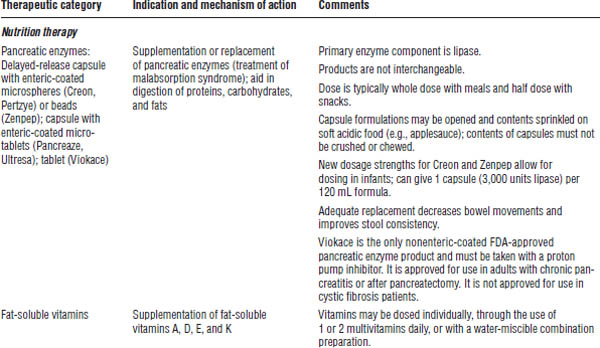
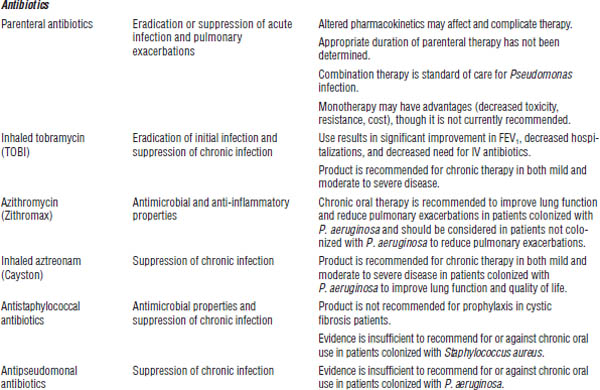
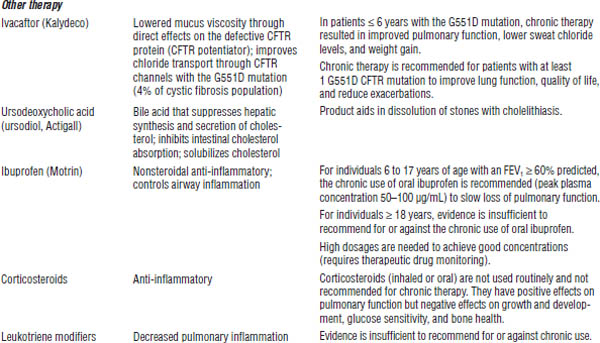
DNA, deoxyribonucleic acid.
a. Other therapies include bronchodilators, inhaled tobramycin, recombinant human DNase, and N-acetylcysteine.
Burkholderia and Stenotrophomonas species are commonly resistant. Follow culture and sensitivity results. Antibiotics that may be effective include trimethoprim-sulfamethoxazole (Septra, Bactrim), chloramphenicol, ceftazidime [Fortaz] (B. cepacia), doxycycline (Vibramycin), and piperacillin [Pipracil] (S. maltophilia).
Patient instructions and counseling
Compliance with therapeutic regimens is important.
Pancreatic enzyme supplementation
■ Give immediately before or during snacks and meals.
■ Capsule may be opened and contents sprinkled on applesauce or other acidic carrier.
■ Contents should not be crushed or chewed.
Aminoglycosides
■ Monitor urine output.
■ Use ibuprofen with caution if dehydration, diarrhea, or decreased oral intake is present.
Ivacaftor (Kalydeco)
■ Take with fat-containing food.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



