Introduction
The management of patent ductus arteriosus (PDA) in the premature neonate is an area of care that is not straightforward. Despite the general paucity of literature available for short- and long-term outcomes in premature infants, there is a respectable amount of evidence when using medications with PDA. However, data are not always clear in terms of what to use, when to treat, or for how long. Although significant morbidities are associated with PDA, they are not proven as cause and effect per se. The physiologic role of the ductus arteriosus (DA) during gestation and the normal transition from intrauterine to extrauterine life in the preterm neonate is reviewed. Understanding how blood flows provides the basis for understanding why a PDA post-delivery could have harmful consequences. Ductal-dependent lesions associated with other congenital heart malformations are not addressed in this chapter.
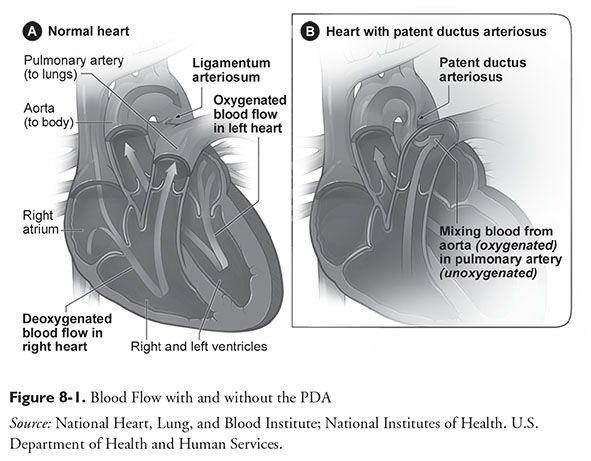
The DA is a large vessel that connects the pulmonary artery to the descending aorta.1 During pregnancy, it shunts the highly oxygenated blood from the placenta past the pulmonary vasculature; this blood can bypass pulmonary circulation because oxygenation occurs in the placenta. Fetal systemic vascular resistance is very low and pulmonary vascular resistance is very high prior to birth, resulting in a left to right flow of blood through the heart. The high pulmonary and very low placental and systemic pressures are one mechanism to keep the antenatal shunt open. After birth, the pulmonary pressures dramatically fall and systemic resistance increases with removal of the placenta, allowing blood to flow through the pulmonary artery to the lungs and then back via the pulmonary vein establishing normal post-natal circulation.
The transition from intrauterine to extrauterine life is quite complex, involving essentially every organ system. Two major areas are the cardiovascular and respiratory systems with support from the endocrine system. A review of all the changes that occur during and following birth is beyond this chapter’s scope, but it is important to realize that most of the information originates from animal research, primarily in sheep or baboons. For an infant to survive post-delivery, lung fluid must be resorbed, surfactant must be made, oxygenation must occur via the alveoli, and the in-utero shunts that facilitate fetal circulation must close.2 Figure 8-1 illustrates blood flow with and without a PDA.
Incidence
The DA’s failure to close increases with decreasing gestational age (GA). It is estimated that approximately 54% of infants born between 22 and 26 weeks will have a PDA, with a 47% incidence in all infants below 28 weeks gestation.3,4 The presence of respiratory distress syndrome also increases the risk of a ductus remaining patent. In term infants, functional closure of the DA occurs within 24 hours and within 1 to 5 days in the majority of preterm infants.
Pathophysiology
Many factors contribute to the DA remaining open after birth. Lower GA is the largest risk factor. Other factors that increase the risk or are associated with PDA include excessive fluid administration, birth asphyxia, congenital syndromes (including congenital rubella syndrome, Trisomy 13, and Trisomy 18), congenital heart disease, and respiratory distress syndrome (RDS). PDA is found in 60 to 70% of infants with certain congenital syndromes.1 Data in both preterm animals and humans, which add yet another layer of complexity, demonstrate that PDA is linked not only to RDS but also to surfactant administration. The development of a significantly hemodynamic PDA has been described by multiple authors and is likely related to the mechanism of the surfactant. When administered, surfactant causes lung compliance to increase resulting in a drop of pulmonary vascular resistance, allowing blood to shunt back across the DA.5–8
The histology of the DA is quite different from the two vascular structures it connects. The DA’s medial layer is composed of longitudinal and spiral layers of smooth muscle fibers within concentric layers of elastic tissue, whereas the medial layers of the aorta and pulmonary artery are primarily concentric elastic tissue.9 When a term infant is born, the sharp increase in the partial pressure of oxygen (PaO2) and reduction in prostaglandin E2 (PGE2) and prostacyclin I2 (PGI2) begin the process of ductal constriction.10 This extreme ductal constriction results in tissue ischemia and cell death, followed by recruitment of inflammatory cells and growth factors that cause anatomical changes to form the ligamentum arteriosum as a permanent closure where the DA was located. A newer mechanism also describes the importance of platelet activation in the mouse model, theorizing that platelet coagulation also impacts successful and permanent closure of the DA.11
It is theorized that the tissues in the preterm DA are not as sensitive to the change in PaO2 after birth and that circulating levels of PGE2 and PGI2 do not drop off as quickly. Further, low platelets have been identified as an independent risk factor for failure of a PDA to close or respond to pharmacologic treatment.12 Even though the placenta is removed (the major source of prostaglandin and prostacyclin production) and oxygenation rises post-birth, the ductus is less likely to close and/or stay closed over the course of the first or second week of postnatal adaptation.
It has also been found that late-onset (after 2 weeks) sepsis can “reopen” the DA. This is due to the fact that anatomic closure of the PDA has not yet occurred in the preterm infant. The sepsis syndrome increases the circulating levels of tumor necrosis factor alpha and prostaglandins,which allow blood to flow again in a left to right direction.4
Importance of PDA in Neonates
In the preterm infant, a persistently open DA has been associated with significant morbidity and disease states that can have high mortality. Although not clearly linked as cause and effect, observational trials have reported that babies with PDA have a higher incidence of bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and intraventricular hemorrhage (IVH).13 If left to right shunting continues, blood can back up into the pulmonary vasculature and cause pulmonary edema. At the same time, systemic blood flow is compromised and ischemic damage may occur. Therefore, it would seem logical that if PDA is successfully treated, then these adverse outcomes should be reduced. However, the available outcomes data are quite heterogeneous, and only prophylactic use of indomethacin has been shown to reduce the rate of morbidity (i.e., IVH). Furthermore, no improvement of neurodevelopmental outcomes has been proven in studies, even with the reduction in severe IVH (defined as grade III or IV) with prophylactic indomethacin. Rates of BPD—another major focus of NICU outcomes in preterm neonates—have not been reduced with any PDA treatment strategy.
Non-Steroidal Anti-Inflammatory Drug Therapy
Two nonsteroidal anti-inflammatory drugs (NSAIDs), indomethacin and ibuprofen lysine, have been studied for closing or preventing PDA. The primary mechanism of action is an inhibition of circulating prostaglandins via inhibition of the cyclooxygenase pathways. In 1985, indomethacin was approved for use and is indicated to “close a hemodynamically significant PDA in premature infants weighing between 500 and 1,750 g when 48 hours of usual medical management (e.g., fluid restriction, diuretics, respiratory support) is ineffective.”15 Ibuprofen lysine labeling is indicated to “close a clinically significant PDA in premature infants weighing between 500 to 1,500 g who are no more than 32 weeks’ GA when usual medical management with diuretics, fluid restriction, and respiratory support is ineffective.”16
Many trials compare oral or intravenous (IV) ibuprofen to placebo; however, some studies directly compare ibuprofen to indomethacin. The largest meta-analysis available concludes that there is no difference in successful closure of a PDA between the two medications, but ibuprofen may have fewer adverse effects on renal and cerebral blood flow and possibly fewer gastrointestinal (GI) side effects.17 Although fewer negative effects are cited with ibuprofen, Abdel-Hady et al. also note that long-term consequences as a result of these differences have not been seen. In cases where neonates have moderately impaired renal function defined as urine output greater than 0.6 to 1 mL/kg/hour, ibuprofen is preferred in some units based on the above data, even though long-term data have yet to substantiate whether these acute side effects result in long-term harm. In vitro studies have demonstrated that ibuprofen displaces bilirubin from albumin and increases the plasma levels of unbound bilirubin. However, in vivo studies did not show similar effects in preterm infants treated with the current recommended doses of 10-5-5 mg/kg/day particularly if total bilirubin levels were below 10 mg/dL before treatment.18
Finally, both medications have also been evaluated (but only in subgroup analyses) for prevention of pulmonary hemorrhage, and only indomethacin was beneficial.
Ultimately, either agent has been found to close a PDA up to 75% of the time in clinical trials. In day-to-day practice, successful closure rates are lower. It is important for the pharmacist to be cognizant that successful closure becomes less likely as time progresses, in part because levels of circulating prostaglandin (the target of NSAID therapy) are reduced with postnatal adaptation. It is suggested that PDA closure with NSAIDs is not a reasonable option beyond 3 to 4 weeks of age, as success is very unlikely; some centers consider surgical closure beyond 1 month of age.1
Dosing
Dosing of indomethacin for PDA closure consistently has an initial dose of 0.2 mg/kg and is followed by 0.1 mg/kg, 0.2 mg/kg, or 0.25 mg/kg for post-natal age less than 48 hours, 2 to 7 days, or greater than 7 days, respectively. The dosing intervals vary between 12 to 24 hours depending on the clinical situation. The success of ductal closure is reduced as the infant approaches 2 weeks of age, so higher doses and more frequent administration may be considered. Conversely, if there are baseline renal function concerns because of reduced urine output (less than 1 to 2 mL/kg/hour), less frequent dosing may be used and repeat laboratory tests may be performed to assess tolerability with each daily dose.15 Finally, some NICUs evaluate for ductal closure after each indomethacin dose and, if successful, do not administer additional doses.
Ibuprofen has been studied in a limited number of infants at higher doses; however, unlike indomethacin, the frequency is always every 24 hours. Standard dosing is 10 mg/kg followed by 5 mg/kg at 24 and 48 hours after the first dose. A handful of trials have evaluated higher dosing with 20 mg/kg followed by 10 mg/kg at 24 and 48 hours.20,21 These trials reported a higher success rate of DA closure with no increase in adverse effects. The former was a pre-symptomatic design and the latter a retrospective prophylactic design; however, the total number of neonates remains small.
Repeating or extending the course of either medication is an option and fairly common. Outcomes data are heterogeneous in terms of additional benefit and adverse effects. Clinicians may express some concern, as one meta-analysis of five randomized trials found no increased success of PDA closure rate with an extended duration of indomethacin treatment but an increase in NEC.22 Another trial found that 44% of infants who did not respond to the first course will close their DA with a second course without additional negative sequelae.23 At this time, research is limited on evaluating a treatment course beyond three doses of ibuprofen because most authors have investigated higher doses on a mg/kg/day basis as noted above.24,25 The percentage of DA that will close with a repeated or extended course is consistently smaller than the closure in the initial treatment course, but surgical ligation (discussed below) is generally avoided in most NICUs until after the DA fails to close with two treatment courses of medication. In many units, surgical ligation requires an infant to be transferred to another facility altogether; thus, two or sometimes three courses of medication may be administered.
Although uncommon, two small studies evaluated extending the duration of indomethacin administration (continuous infusion versus traditional 30-minute bolus infusion). This approach, based on the theory that an extended infusion time might reduce the vasoconstrictive and ischemic effects of bolus administration, found no significant differences in terms of PDA, NEC, IVH, or mortality; however, the restriction of blood flow was less in the cerebral, renal, and mesenteric arteries with the extended infusion time.26 A similar trial with ibuprofen compared a continuous infusion (over 24 hours) with bolus dose of labeling dosing (10-5-5 mg/kg/day). This prospective, double-dummy trial of more than 100 randomized infants found higher PDA closure rates, fewer NEC symptoms, and fewer surgical ligations in the continuous infusion arm.27
Side Effects, Contraindications, and Drug Interactions
The serious side effects of NSAIDs relate to their mechanism of action. Reduced cerebral, GI, and renal blood flow can result in multiple morbidities. IVH or periventricular leukomalacia, NEC, and transient decreases in urinary output and increases in blood urea nitrogen and creatinine can result. Although reported less frequently with ibuprofen than indomethacin, labeling for both agents include these adverse events. Platelet dysfunction is also a known side effect of all NSAIDs; thus, other bleeding complications or even disseminated intravascular coagulation has been reported. If urine output drops below 0.6 mL/kg/hour, subsequent doses of either agent should be held.15,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree