where P is the partition coefficient and the subscripts indicate the solvent phase. Note that it would be equally possible to define
(3.2)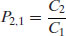
In a physiological environment drug substances partition from an aqueous phase to numerous and heterogeneous lipophilic phases (typically cell membranes). It would be complex to develop an analytical method that allowed measurement of partitioning between such complex phases and so a simple solvent model is usually used instead (typically n-octanol, which is taken to mimic the short-chain hydrocarbons that make up many biological lipid bilayers). Thus, a partition coefficient can be written to represent the distribution of a solute between water (w) and n-octanol (o):
(3.3)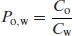
Again, the following could be defined:
(3.4)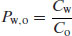
However, by convention Po,w is the standard term (easily remembered as organic phases usually sit above water, since they are less dense).
Study question 3.1 What range will the values of Po,w have for lipophilic and hydrophilic drug substances?
For simplicity it will be assumed that only un-ionised species can partition into the organic phase1 so the partition coefficient as defined above applies only if (a) the drug substance cannot ionise or (b) the pH of the aqueous phase is such that the drug substance is completely un-ionised. If the drug substance has partially ionised in the aqueous phase and partitioning is measured experimentally then the parameter measured is the distribution coefficient, D:
(3.5)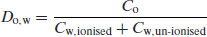
The partition coefficient and the distribution coefficient are related by the fraction of solute un-ionised (fun-ionised):
(3.6)
- Partition coefficients are determined between water and an organic (usually n-octanol) phase.
- Partition coefficients can be defined as o/w or w/o.
- By convention, o/w values are used.
- For lipophilic drugs, P > 1 or log P is positive.
- For hydrophilic drugs, P < 1 or log P is negative.
- Ionised drug has low affinity for organic phases.
- Experimental measurement of partitioning for a drug that ionises results in a distribution coefficient.
Note that partition coefficients may be defined between any organic phase and water. n-Octanol is a common choice but it is by no means either the best choice or the only choice, especially if partition coefficients are estimated using chromatographic methods. The Collander equation relates the partition coefficients for water with a range of organic solvents:
(3.7)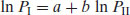
where the subscripts I and II refer to water and the organic solvent respectively and a and b are constants. Beezer et al. (1987) show that while the constant a has no fundamental significance, the value of b is reflective of differences between organic phases and is useful in scaling solvent behaviour to biological membrane properties.
3.2.1 Effect of partitioning
Leo, Hansch and Elkins (1971) review the use of partition coefficients in pharmaceutical development. The main effect, from the perspective of a drug substance, is on absorption in vivo. Crossing biological membranes (assuming no active transport mechanisms are available) essentially involves a series of partitioning steps between organic and aqueous phases and so a balance of lipophilicity and hydrophilicity is required to prevent accumulation of the drug substance in one particular phase. The optimum balance depends upon the site of absorption within the body. Buccal and GI tract absorption require a relatively high hydrophilicity, while a low value is beneficial in aiding uptake across the blood–brain barrier.
Partitioning will also affect solubility when two phases are in contact with each other and, since ionic species have little affinity for organic phases, the bioavailability of salt forms may be limited. From an analytical perspective partitioning also underpins solvent extraction techniques.
3.2.2 Determination of log P
Values for log P can be determined experimentally or can be estimated using group additivity functions. For the latter approach there are numerous computer models and simulation methods available and selection will reduce to personal choice and familiarity, and so these models will not be considered here. This text will focus on experimental determination. It is clear, however, that there is much value to be gained by comparing calculated and experimentally determined log P values; nor can the value of the calculational approach be underestimated when selecting a lead candidate from a compound library when it would simply not be either possible or practicable to measure the partitioning behaviour of the many thousands of compounds available.
3.2.2.1 Shake-flask method
Assuming a UV–visible assay is available, then the shake-flask method is a quick, simple and near universally applicable way of determining the partition coefficient. Prior to measurement, the solvents to be used (normally water, or buffer, and n-octanol) should be mixed with each other and allowed to reach a position of equilibrium. This is because each solvent has a significant solubility in the other (n-octanol in water: 4.5×10−3 M; water in n-octanol: 2.6 M, Weber, Chin and Rice, 1986). There can be a significant effect of saturating water with n-octanol on the solubilities of compounds. Chiou, Schmedding and Manes (1982) report an increase in solubility of DDT and hexachlorobenzene of 160 and 80% respectively in water saturated with n-octanol compared with the solubilities in pure water.
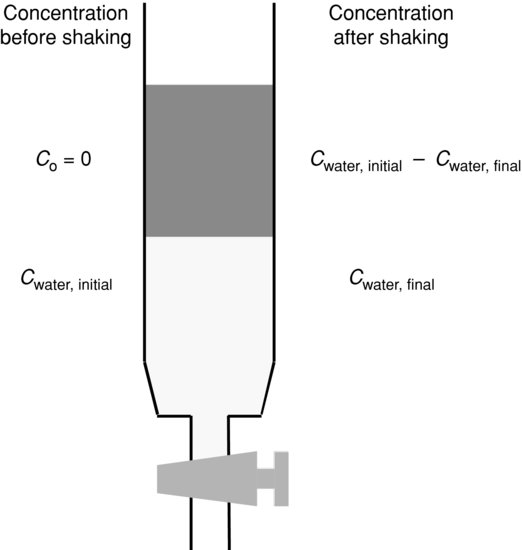
Following preparation of the solvent phases, the drug substance is dissolved in the aqueous phase to a known concentration. This could be the solubility (indeed, the solution used to determine solubility can subsequently be used in the partitioning experiment) because the higher the concentration the more reliable the assay. Equal volumes of aqueous drug solution and n-octanol are then mixed in a separating funnel. The mixture should be sha- ken vigorously for a period of time (usually 30 min, to maximise the surface area of the two solvents in contact with each other) while the drug substance partitions. The phases are allowed to separate (5 min) and then the concentration of drug substance remaining in the aqueous phase is determined with the UV–visible assay already developed (Figure 3.1). By difference, the concentration of drug substance in the n-octanol phase is known:
(3.8)
When the partition coefficient heavily favours distribution to the n-octanol phase then a smaller volume of n-octanol can be added, since this will increase the concentration in the aqueous phase at equilibrium and reduce the error in the analytical determination of the concentration. However, the calculation for partition coefficient needs to be corrected to account for the different volumes. Assuming a 1:9 n-octanol:water ratio, then Equation (3.1) becomes
(3.9)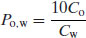
Study question 3.2 What are the drawbacks of the shake-flask method?
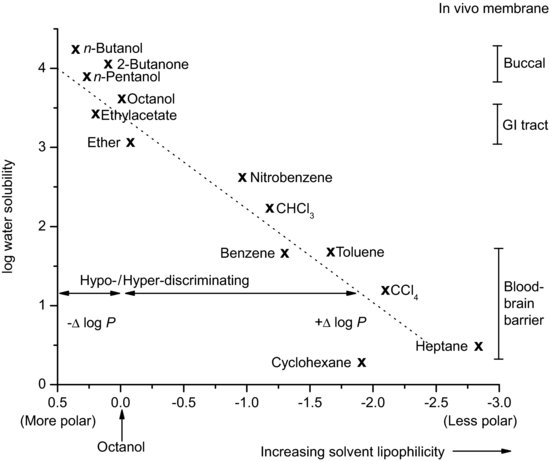
n-Octanol may also not be the best organic phase. Hexane or heptane can be used as an alternative, although they will give different partition coefficient values from n-octanol and are also considered to be less representative of biological membranes because they cannot form any hydrogen bonds with the solute. Where the aim of the experiment is to differentiate partitioning between members of a homologous series the organic phase can be varied in order to maximise discrimination. n-Butanol tends to result in similar partition values for a homologous series of solutes, while heptane tends to exaggerate differences in solute lipophilicity. Solvents that are more polar than n-octanol are termed hypodiscriminating and those that are less polar than n-octanol are termed hyperdiscriminating. Hyperdiscriminating solvents reflect more closely transport across the blood–brain barrier while hypodiscriminating solvents give values consistent with buccal absorption. n-Octanol tends to reflect absorption from the GI tract, which is why it is the default option. The discriminating powers of a range of common solvents, relative to n-octanol, are shown in Figure 3.2.
Figure 3.2 Discriminating power of various partitioning solvents (redrawn from Wells (1988), with permission from John Wiley & Sons, Inc.).