7 Parenteral nutrition
Introduction
Malnutrition
Nutrition screening
Routine screening is recommended by the Malnutrition Advisory Group of the British Association of Parenteral and Enteral Nutrition (BAPEN). This group has worked to promote awareness of the clinical significance of malnutrition and has produced guidelines to monitor and manage malnutrition. A range of screening criteria and tools have been developed and refined to assess nutritional status. Examples include the relatively simple and reproducible body mass index tool with consideration of other key factors (Table 7.1), and the BAPEN ‘MUST’ tool (Malnutrition Universal Screening Tool; BAPEN, 2003). Body weight should not be used in isolation; significant weight fluctuations may reflect fluid disturbances, and muscle wasting may be due to immobility rather than undernutrition. More complex anthropometry measurements are sometimes indicated to track changes.
Table 7.1 Body mass index as a screening tool
| BMI (kg/m2) | BMI category |
|---|---|
| <18.5 | Underweight |
| 18.5–25 | Ideal BMI |
| 25–29.9 | Overweight |
| >30 | Obese |
Body mass index (BMI) = weight (kg)/height (m)2
Indications for parenteral nutrition (PN)
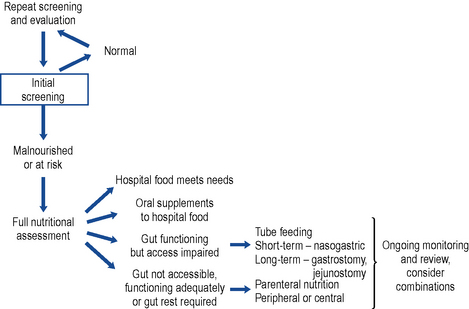
A decision pathway can be followed to guide initial and ongoing nutritional support. While many are published, a locally tailored and regularly updated pathway is favoured. A useful starting point may be found at Fig. 7.1.
Nutrition support teams
A report published by the Kings Fund Report (1992) highlighted the issue of malnutrition both in the hospital and home setting. The findings led to the development of the British Association of Enteral and Parenteral Nutrition (BAPEN) and nutrition support teams throughout the UK. These multidisciplinary nutrition support teams comprise a doctor, nurse, pharmacist and dietitian. They function in a variety of ways, depending on the patient populations and resources. In general, they adopt either a consultative or an authoritative role in nutrition management. Many studies have shown their positive contribution to the total nutritional care of the patient through efficient and appropriate selection and monitoring of feed and route.
Components of a PN regimen
In addition to water, six main groups of nutrients need to be incorporated in a PN regimen (Table 7.2). The aim is to provide appropriate sources and amounts of all the equivalent building blocks in a single daily admixture.
Table 7.2 Oral and equivalent parenteral nutrition source
| Oral diet | Parenteral nutrition source |
|---|---|
| Water | Water |
| Protein | L-amino acids mixture |
| Carbohydrate | Glucose |
| Fat with essential fatty acids | Lipid emulsions with essential fatty acids |
| Vitamins | Vitamins |
| Minerals | Trace elements |
| Electrolytes | Electrolytes |
Water volume
Water is the principal component of the body and accounts for approximately 60% and 55% of total body weight in men and women, respectively. Usually, homeostasis maintains appropriate fluid levels and electrolyte balance, and thirst drives the healthy person to drink; however, some patients are not able physically to respond by drinking and so this homeostasis is ineffective. There is risk of over- or underhydration if the range of factors affecting fluid and electrolyte balance is not fully understood and monitored. In general, an adult patient will require 20–40 ml/kg/day fluid; however, Table 7.3 describes other factors that should be considered in tailoring input to needs.
Table 7.3 Factors affecting fluid requirements
| Consider increasing fluid input | Consider reducing fluid input |
|---|---|
| Signs/symptoms of dehydration | Signs/symptoms of fluid overload |
| Fever: increased insensible losses from lungs in hyperventilation and from skin in sweating. Allow 10–15% extra water per 10°C above normal | High humidity: reduced rate of evaporation |
| Acute anabolic state: increased water required for increased cell generation | Blood transfusion: volume input |
| High environmental temperature or low humidity: increased rate of evaporation | Cardiac failure: may limit tolerated blood volume |
| Drug therapy: assess volume and electrolyte content of infused drug | |
| Abnormal GI loss (vomiting, wounds, ostomies, diarrhoea): consider both volume loss and electrolyte content | |
| Burns or open wound(s): increased water evaporation | |
| Renal failure: fluid may accumulate so reduce input accordingly or provide artificial renal support | |
| Blood loss: assess volume lost and whether replaced by transfusion, colloid, crystalloid |
Amino acids
To balance the patient’s amino acid requirements and the chemical characteristics of the amino acids (solubility, stability and compatibility), a range of commercially available licensed solutions has been formulated containing a range of amino acid profiles (Table 7.4). Aminoplasmal®, Aminoven, Synthamin® and Vamin® are designed for adult patients. The amino acid profiles of Primene® and Vaminolact® are specifically tailored to neonates, infants and children (reflecting the amino acid profile of maternal cord blood and breast milk, respectively).
Table 7.4 Examples of amino acid and consequential nitrogen content of licensed amino acid solutions available in the UK
| Name | Nitrogen content (g/L) | Electrolytes present |
|---|---|---|
| Aminoplasmal® 5% E | 8 | Potassium, magnesium, sodium and dihydrogen phosphate |
| Aminoplasmal® 10% | 16 | |
| Aminoven® 25 | 25.7 | |
| Glamin® | 22.4 | |
| Hyperamine® 30 | 30 | Sodium |
| Primene® 10% | 15 | |
| Synthamin® 9 | 9.1 | Potassium, magnesium, sodium and acid phosphate |
| Synthamin® 9 EF | 9.1 | |
| Synthamin® 14 | 14 | Potassium, magnesium, sodium and acid phosphate |
| Synthamin® 14 EF | 14 | |
| Synthamin® 17 | 17 | Potassium, magnesium, sodium and acid phosphate |
| Synthamin® 17 EF | 17 | |
| Vamin® 9 Glucose | 9.4 | Potassium, magnesium, sodium and calcium |
| Vamin® 14 | 13.5 | Potassium, magnesium, sodium and calcium |
| Vamin® 14EF | 13.5 | |
| Vamin® 18EF | 18 | |
| Vaminolact® | 9.3 |
Energy
Many factors affect the energy requirement of individual patients and these include age, activity and illness (both severity and stage). Predictive formulae can be applied to estimate the energy requirement, for example, the Harris Benedict equation or the more commonly used Schofield equation, which is shown below in Table 7.5.
| Age (years) | Male | Female |
|---|---|---|
| 15–18 | BMR = 17.6 × weight (kg) + 656 | BMR = 13.3 × weight (kg) + 690 |
| 18–30 | BMR = 15.0 × weight (kg) + 690 | BMR = 14.8 × weight (kg) + 485 |
| 30–60 | BMR = 11.4 × weight (kg) + 870 | BMR = 8.1 × weight (kg) + 842 |
| >60 | BMR = 11.7 × weight (kg) + 585 | BMR = 9.0 × weight (kg) + 656 |
BMR, basal metabolic rate.
Dual energy
While effectively maintaining nitrogen balance, lipid inclusion is seen to confer a number of advantages (Box 7.1). Some patients, notably long-term home patients, do not tolerate daily lipid infusions and need to be managed on an individual basis. Depending on the enteral intake and nutritional needs, lipids are prescribed for a proportion of the days. A trial with the newer generation lipid emulsions may be appropriate.
Glucose
Glucose is the recommended source of carbohydrate (1 g anhydrous glucose provides 4 kcal). Table 7.6 indicates the energy provision and tonicity for a range of concentrations. Glucose 5% is regarded as isotonic with blood. The higher concentrations cause phlebitis if administered directly to peripheral veins and should, therefore, be given by a central vein or in combination with compatible solutions to reduce the tonicity.
Table 7.6 Energy provision and tonicity of glucose solutions
| Concentration (w/v) | Energy content (kcal/L) | Osmolarity (mOsmol/L) |
|---|---|---|
| 5% | 200 | 278 |
| 10% | 400 | 555 |
| 20% | 800 | 1110 |
| 50% | 2000 | 2775 |
| 70% | 2800 | 3885 |
Lipid emulsions
Typically, patients receive up to 2.5 g lipid/kg/day. For practical compounding reasons, and assuming clinical acceptance, this tends to be rounded to 100 g or 50 g. Details of lipid emulsions available within the UK can be found in Table 7.7.
Table 7.7 Examples of licensed lipid emulsions available in the UK
| Lipid emulsion type | Details of products with kJ per litre |
|---|---|
| Soybean oil | Intralipid® 10% (4600), 20% (8400), 30% (12600) |
| Purified olive oil/soybean oil | ClinOleic® 20% (8360) |
| Medium chain triglycerides/soybean oil | Lipofundin® MCT/LCT 10% (4430), 20% (8000) |
| Purified structured triglycerides | Structolipid® 20% (8200) |
| Omega-3-acid triglycerides/soybean oil/medium chain triglycerides | Lipidem® (7900) |
| Highly refined fish oil | Omegaven® (4700) |
| Fish oil/olive oil/soybean oil/medium chain triglycerides | SMOFLipid® (8400) |
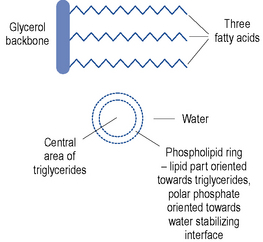
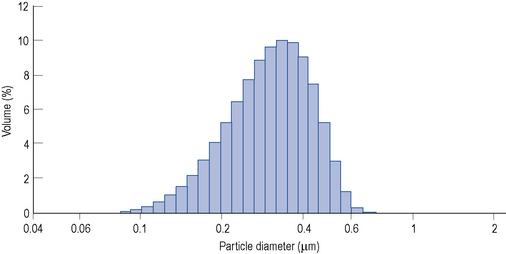
Lipid emulsions are oil-in-water formulations. Figure 7.2 shows the structure of triglycerides (three fatty acids on a glycerol backbone) and a lipid globule, stabilised at the interface by phospholipids. Ionisation of the polar phosphate group of the phospholipid results in a net negative charge of the lipid globule and an electromechanically stable formulation. The lipid globule size distribution is similar to that of the naturally occurring chylomicrons (80–500 nm), as indicated in Fig. 7.3.
Lipid clearance monitoring is particularly important in patients who are at risk of impaired clearance, including those who are hyperlipidaemic, diabetic, septic, have impaired renal or hepatic function or are critically ill (Crook, 2000).
Micronutrients
By the time a patient starts PN, they may have already developed a deficiency of one or more essential nutrients. By the time a specific clinical deficiency is observed, for example, depigmentation of hair in copper deficiency or skin lesions in zinc deficiency, the patient will already have tried to compensate to maintain levels, compromised intracellular enzyme activity and antioxidant systems and expressed non-specific symptoms such as fatigue and impaired immune response. A summary of factors that affect micronutrient needs is presented in Box 7.2.
Box 7.2 Factors affecting micronutrient requirements
Baseline nutritional state on starting parenteral nutrition
Measuring blood levels of vitamins and trace elements in acutely ill patients is of limited value. It is recommended that these are measured every 1–6 months depending on levels, and in patients at home on PN (NICE, 2006). Deficiency states are clinically significant but, with non-specific symptoms, they are often difficult to diagnose.
The micronutrients naturally fall into two groups: the trace elements and vitamins. Micronutrients should be added to all PN infusions under appropriate, controlled, environmental conditions prior to administration (NICE, 2006).
Trace elements
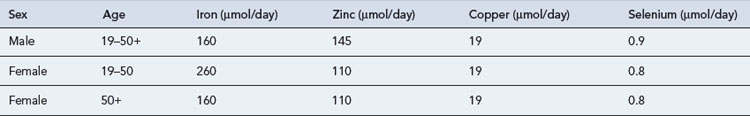
Trace elements are generally maintained at a relatively constant tissue concentration and are present to a level of less than 1 mg/kg body weight. They are essential; deficiency results in structural and physiological disorders which, if identified early enough, can be resolved by re-administration. Ten essential trace elements are known: iron, copper, zinc, fluorine, manganese, iodine, cobalt (or as hydroxycobolamin), selenium, molybdenum and chromium. Adult reference ranges for daily requirements of trace metals can be found in Table 7.8. Currently two preparations are commercially available for adults (Decan®, Additrace®) together with a single paediatric preparation (Peditrace®).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree