Papillomas are discrete benign tumors of the epithelium of mammary ducts. They arise most often from lactiferous ducts in the central part of the breast, but they can occur peripherally in any quadrant.
Intracystic papilloma is the designation applied to a papilloma in a cystically dilated duct. Large, complex papillomas that have a cystic component have sometimes been referred to as
papillary cystadenomas, whereas solid, noncystic papillomas have been variously classified as
ductal adenoma, solid papilloma, or
adenomyoepithelioma (
AME). A
solitary papilloma is a single discrete papillary tumor in one duct.
Multiple papillomas usually occur in contiguous branches of the ductal system. Solitary papillomas are more common than multiple papillomas. A distinction must be made between intraductal papilloma and papillomatosis (epitheliosis). The latter terms are used to describe usual (conventional) ductal hyperplasia (UDH), the common form of ductal hyperplasia, which may coexist with solitary or multiple papillomas.
Clinical Presentation
In 1951, Haagensen et al.
1 reviewed a series of 367 patients with benign intraductal papillary breast lesions who had been treated at Presbyterian Hospital in New York between 1916 and 1941. After excluding 243 patients with “microscopic papillomas,” 14 with incomplete data, and 2 with a papilloma and unrelated nonpapillary carcinoma, the authors concentrated on describing 108 patients treated for gross, benign intraductal papillomas. Microscopic papillomas were characterized as “small multiple papillary projections, with or without fibrous cores, which project into the ducts and cysts of chronic cystic mastitis.” By contrast, gross papilloma was defined as a “definite disease entity in which one or more papillomas grow within a relatively localized portion of a duct, or in several adjacent ducts, and attain sufficient size to fill up the duct and become evident grossly.”
The study revealed some now well-established features of duct papilloma. The majority (75%) were located in the central part of the breast. Discharge, bloody or nonbloody, was the primary symptom in 72% of cases, but it was less commonly seen with peripheral lesions (29%) than with those in a central duct (86%). Lesions that caused nipple discharge, whether central or peripheral, tended to have a frond-forming papillary configuration, whereas a more solid growth pattern was observed in tumors not associated with a discharge.
Solitary papillomas may occur at any age from infancy to the ninth decade, but they are most frequent in the sixth and seventh decades of life. The tumors usually occur in the subareolar region and come to attention because of a discharge from the nipple. Women with multiple papillomas tend to be younger than women with solitary papillomas and most often present in their 40s and early 50s. Multiple papillomas develop peripherally more often than centrally and typically present as a palpable lesion. Papillomas may occur more frequently in African American women than in women of other ethnic origins.
2Papillomas have developed in unusual settings. One report
3 documented the growth of a papilloma in residual breast tissue following a transverse rectus abdominus muscle (
TRAM) flap reconstruction. Two publications
4,5 describe the presence of papillomas in ectopic breast tissue in axillary lymph nodes (ALNs) of women with mammary papillomas.
Although papillomas afflict women almost exclusively, the literature contains reports of 26 papillomas in men. Volmer
6 lists the cases described prior to 1985. In 2006, Yamamoto et al.
7 tabulated clinical details of 10 additional cases, and five other examples
8,9,10,11,12 have also been published. The patients range from 7 months
13 to 82 years
14 of age, the range seen in women. Two patients presented during their early teenage years,
8,15 and the author has examined a papilloma from a 15-year-old boy. The presence of several cases in adolescent boys seems a curious coincidence, which
might merit further attention. Symptomatic intervals as long as 6 years have been noted.
16 Two patients reported longterm use of phenothiazines.
7,17 One of these patients had an elevated serum prolactin level,
7 and the papillary epithelium in the other case
17 exhibited secretory changes.
Nipple discharge occurs in most patients with a central papilloma. Bloody discharge, more commonly associated with papillary carcinoma, can develop in a papilloma as a result of degenerative changes. A subareolar mass may be palpable in patients with a central solitary papilloma, and a palpable tumor may be the first clinical manifestation of a peripheral papilloma. A papilloma in a 44-year-old man produced a hard, lobulated mass fixed to the chest wall.
10
Imaging Studies
Cystic, solitary papillary tumors may appear well circumscribed on mammography, but the presence of a cystic component is best appreciated by ultrasonography, which seems to be more sensitive than mammography for the detection of papillomas. Francis et al.
18 reported that 44% of mammograms in their study of 36 women with papillomas were described as normal, whereas only 5% of sonograms appeared normal. These authors also cited similar findings presented in earlier publications. Ductography can also demonstrate the presence of a central papilloma in patients who experience a nipple discharge.
19 Magnetic resonance imaging (
MRI) in women with solitary intraductal papillomas and nipple discharge typically reveals duct dilation and the presence of small, oval, smoothly contoured, enhancing intraductal masses,
18,20 but papillomas do not always display these characteristic features. In the study of Kurz et al.,
21 4 of 20 papillomas had irregular shapes, 3 had irregular or spiculated margins, 8 displayed heterogeneous enhancement, and the characteristics of the time-intensity curves varied. After reviewing images from 15 papillomas, Daniel et al.
22 reported that 7 displayed suspicious morphologic findings such as irregular margins or rim enhancement and that 4 were not evident on the
MRI studies.
Cardenosa and Eklund
19 compared the clinical and mammographic findings in patients with solitary and multiple papillomas. The latter group was subdivided into peripheral and central lesions. Most multiple peripheral papillomas were asymptomatic and were detected as a result of mammographic abnormalities, including calcifications, nodules, masses, or various opacities.
After a detailed mammographic and sonographic study of 40 papillary tumors, which were later excised and examined histologically, Lam et al.
23 concluded that “radiologic features are not sufficiently sensitive or specific to differentiate benign from malignant papillary lesions.” By using a combination of both imaging modalities, the authors achieved a sensitivity of 61%, a specificity of 33%, a positive predictive value (
PPV) of 85%, and a negative predictive value (NPV) of 13%.
Gross Pathology
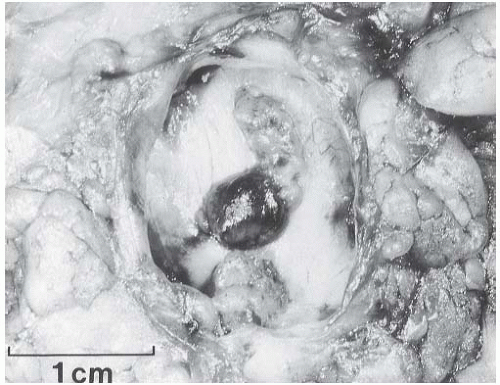
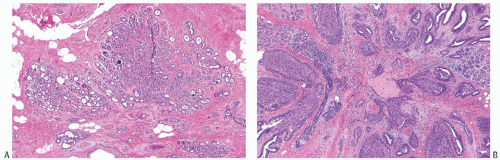
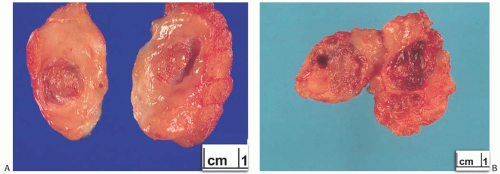
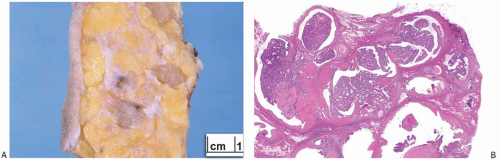
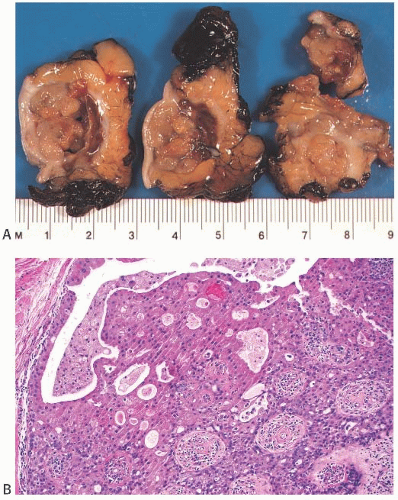
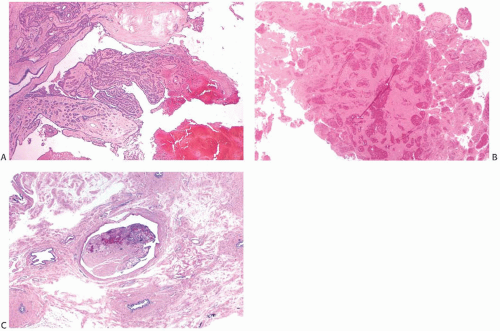
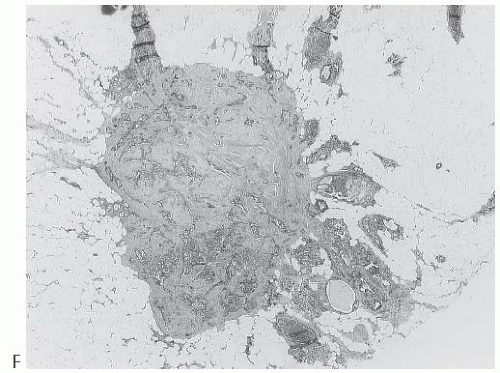
A part of the mass associated with some solitary papillomas is a cyst formed by the dilated duct in which the papilloma grew (
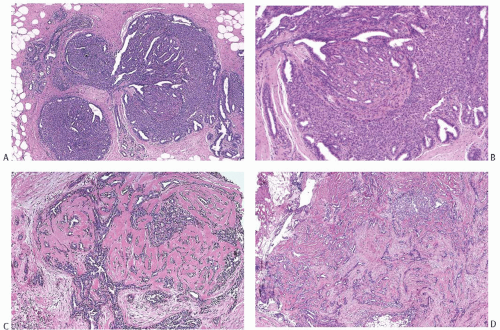
Fig. 5.1). The cyst may contain clear fluid, bloody fluid, or clotted blood. The papilloma usually forms a single mural nodule protruding into the lumen, but occasionally multiple, separate, or aggregated nodules are present (
Fig. 5.2). Some papillomas grow as soft, solid friable masses that obliterate the cystic space.
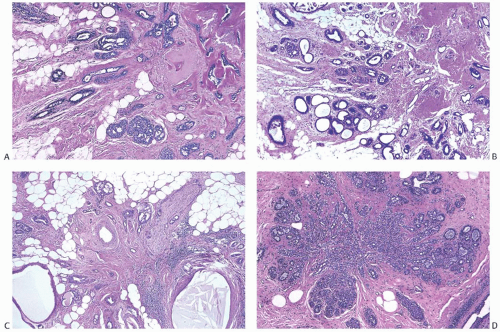
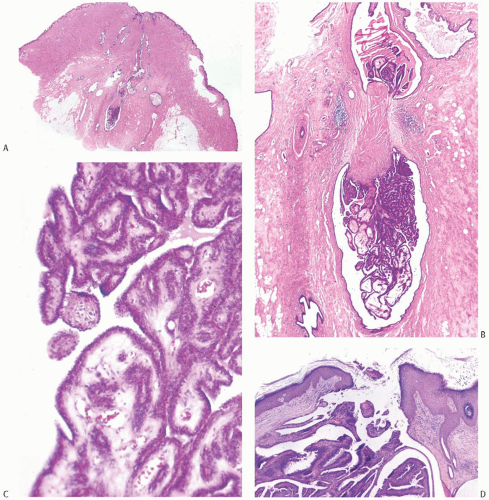
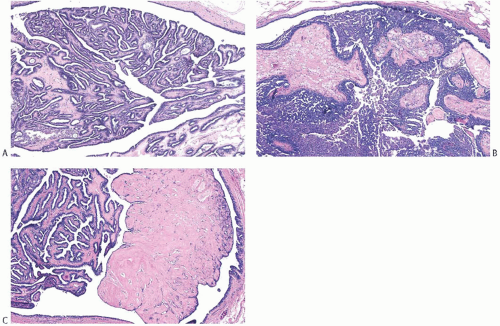
Solitary papillomas are typically bosselated, soft-to-firm tumors that appear gray to reddish brown when examined grossly in the fresh state. The lesions are well circumscribed and appear to be enclosed by a capsule formed by the duct wall and accompanying reactive changes. Multiple associated papillomas can sometimes be appreciated grossly when they form nodules in contiguous dilated ducts (
Fig. 5.3).
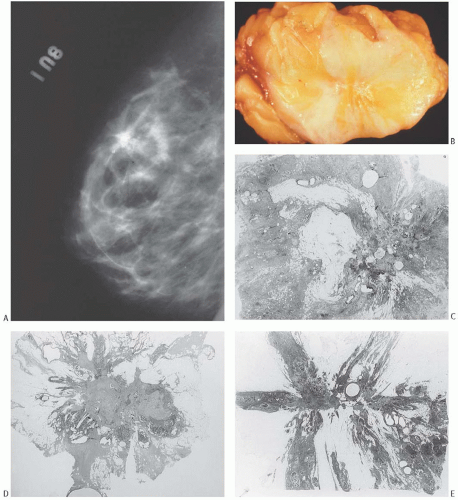
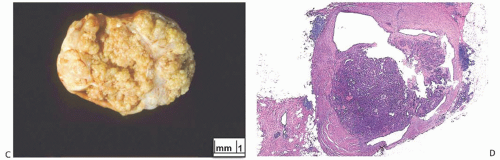
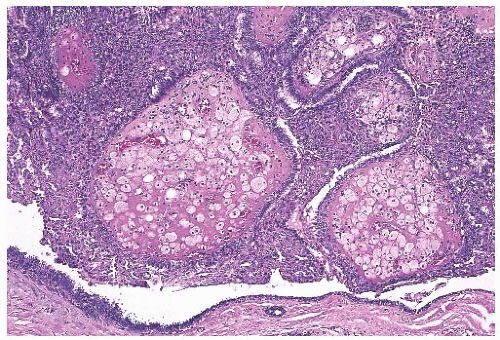
Solitary papillomas may form clinically symptomatic tumors 1 cm or less in diameter in a major lactiferous duct (
Fig. 5.4), but the average size is 2 to 3 cm. Benign cystic papillomas may be larger than 10 cm. Tumors formed by multiple papillomas are typically larger than 2 cm.
1,24
Microscopic Pathology
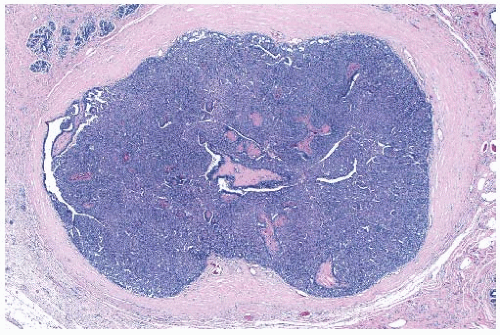
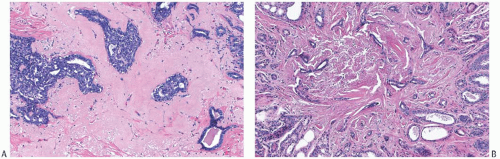
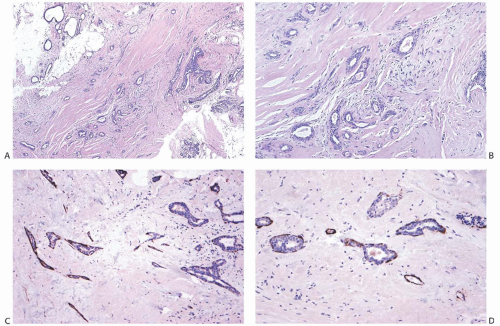
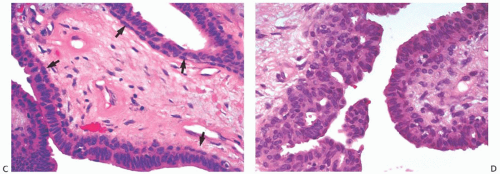
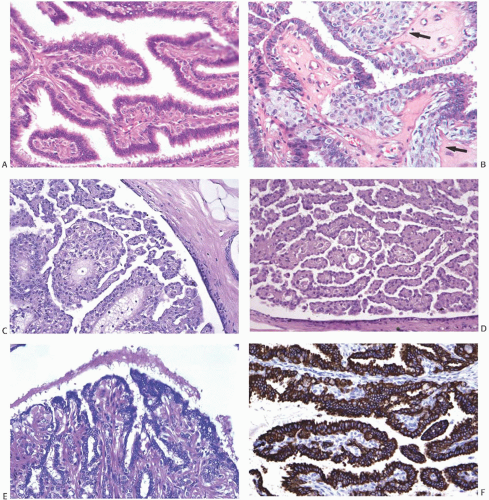
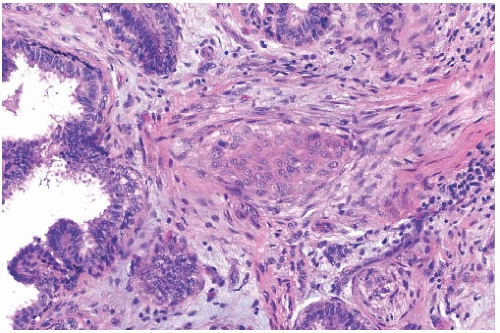
The basic microscopic structure of a papilloma consists of the proliferation of ductal epithelium supported by frondforming fibrovascular stroma. The most orderly form of papilloma consists of branching fronds of stroma supporting a layer of epithelium composed of epithelial and myoepithelial cells (
Fig. 5.5). The epithelial cells are cuboidal to columnar, and they display little pleomorphism, nuclear hyperchromasia, or mitotic activity. The supporting stroma may arise from a single base or from several foci in the duct wall. Epithelium lining the nonpapillary portion of the duct usually exhibits little or no hyperplasia.
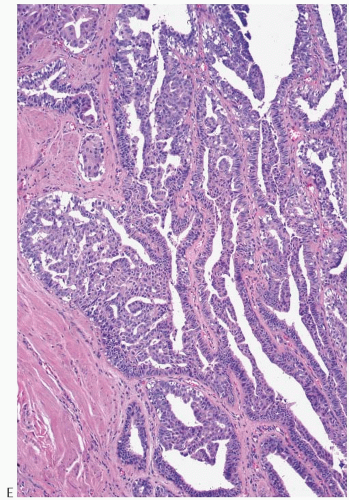
Some papillomas have a more complex structure caused by stromal overgrowth, hyperplasia of the epithelium, or
both processes, resulting in fusion of the papillary fronds (
Fig. 5.6). The most exaggerated form of this process is the solid intraductal papilloma, in which virtually all the space between fibrovascular stalks is filled by solid sheets of proliferative ductal epithelial cells. More often, secondary lumens are formed within the hyperplastic epithelium, resulting in irregular microlumens, micropapillary fronds, focal solid areas, or heterogeneous combinations of these patterns (
Fig. 5.7).
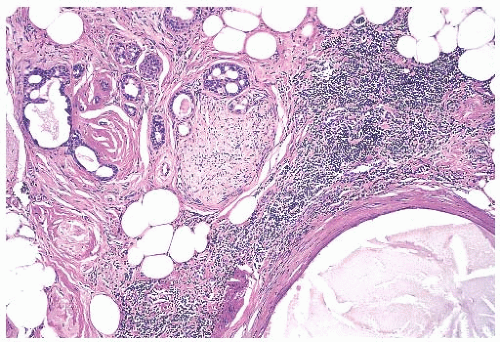
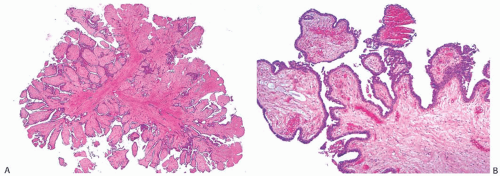
Foci of apocrine metaplasia are not uncommon in papillomas (
Fig. 5.8), and rarely most or all of the epithelium is of the apocrine type. When present in the conventional
papillary configuration, apocrine metaplasia is usually cytologically bland, but apocrine atypia manifested by nuclear pleomorphism and cytoplasmic clearing can be seen in sclerosing papillary tumors. This is especially common when sclerosing adenosis (
SA) is incorporated into a papilloma.
The appearance of the fibrovascular stroma varies considerably among papillomas. In some lesions, the stroma is limited to slender inconspicuous strands consisting of thin-walled capillaries and sparse fibroblasts, collagen, and mononuclear cells and forming a network that supports the voluminous epithelium. The distribution of this stroma stands out especially clearly in sections stained for reticulin, vimentin, basement membrane proteins, or vascular markers such as CD34 and CD31. Expansion of the fibrovascular stroma by accumulated histiocytes occurs in papillomas and is an exceedingly rare finding in papillary carcinoma (
Fig. 5.9).
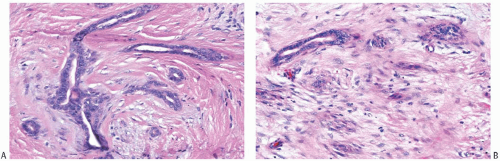
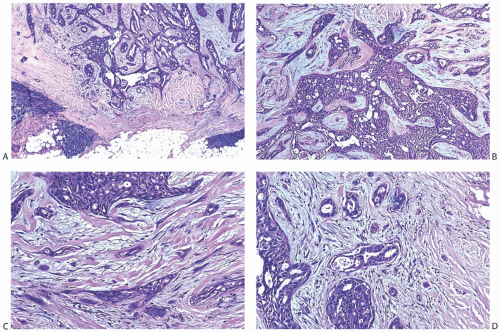
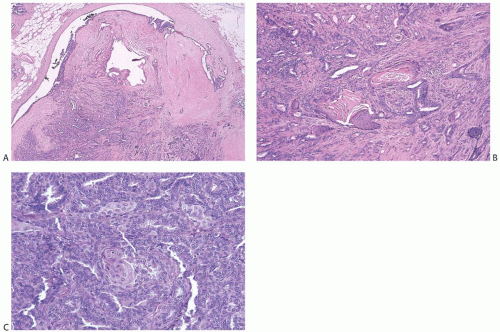
Collagenization of the fibrovascular stroma occurs in some papillomas. The papillary architecture is accentuated when this process is limited to the intrinsic papillary structure. If myofibroblastic proliferation accompanies collagenization of the stroma, the papillary arrangement is likely to become distorted. Epithelial elements entrapped in this stroma may simulate invasive carcinoma within or at the periphery of the lesion (
Figs. 5.10 and
5.11). In the most extreme situations, fibrous sclerosis is so severe as to virtually obliterate the papilloma, reducing it to a nodular scar containing sparse benign glandular elements (
Fig. 5.12). Such a lesion may be difficult to distinguish from a fibroadenoma (
FA). This problem is typified by a case report purported to represent an infarcted
FA in which the illustrations are highly suggestive of a fibrotic and partially infarcted cystic papilloma.
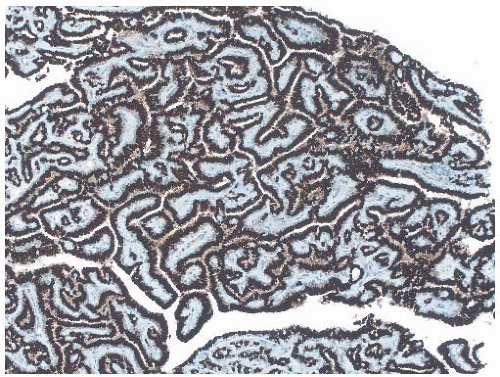
25The epithelium of intraductal papillomas contains a myoepithelial cell layer.
26 Inconspicuous elongated myoepithelial cells with nuclei are flattened along the basement membrane. Hyperplastic myoepithelial cells typically form a prominent layer of cuboidal cells that tend to have relatively clear cytoplasm (
Fig. 5.13). Myoepithelial cells in papillomas can be demonstrated with a variety of immunostains.
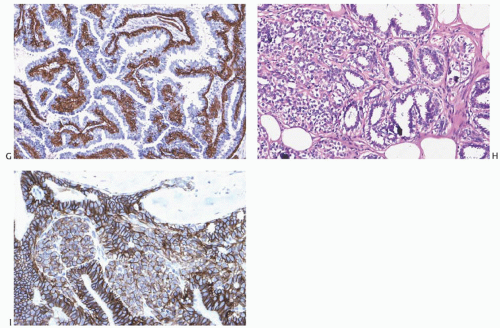
26,27 When immunostaining is done to detect myoepithelial cells, it is necessary to differentiate stromal and myoepithelial reactivity. Myoepithelial cells are immunoreactive for
actin, calponin, myosin heavy chain, CD10, p63, and CK5/6 (
Fig. 5.13). All of these proteins except p63 are cytoplasmic markers, which vary in their proclivity to react with stromal cells. The p63 immunostain localizes in myoepithelial cell nuclei and rarely in the nuclei of epithelial cells in a papillary lesion.
28 Cross-reactivity in stromal cells is not observed. For this reason, it is preferable to include a stain for p63 among those chosen to detect myoepithelial cells. The myoepithelium may become noticeably attenuated and focally undetectable by immunostains in regions of sclerosis in a papilloma. The focal absence of immunoreactive myoepithelium, by itself, is not diagnostic of carcinoma in this setting. When there is marked hyperplasia of myoepithelial cells in a benign papillary tumor, the differential diagnosis includes
AME.
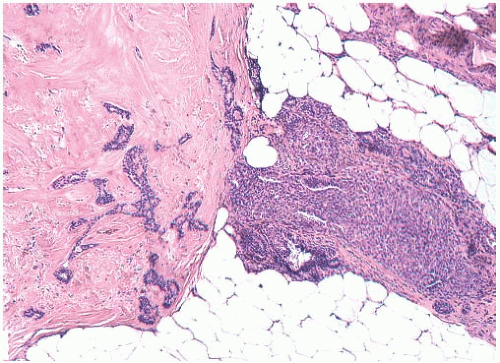
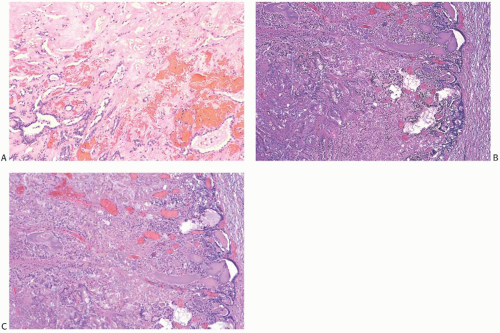
Infarction occurs in solitary and in multiple papillomas (
Figs. 5.14 and
5.15). No specific cause for the necrosis is evident in most cases. The presence of chronic inflammation and hemosiderin in and around many papillomas suggests that these lesions are prone to transient bleeding secondary to ischemia or incidental trauma. Needling procedures can contribute to infarction and cause fresh hemorrhage,
sometimes associated with displaced epithelium. Spontaneous infarction usually involves superficial portions of a papilloma. Rarely, the entire lesion is infarcted either spontaneously or as a consequence of a biopsy. The underlying structure of a fully infarcted papilloma can be demonstrated with a reticulin stain. In some infarcted lesions in which degeneration is not advanced, the structure of the epithelium can be displayed more clearly with immunostains such as cytokeratin (
CK) and p63.
29 There is no procedure for reliably distinguishing between the completely infarcted epithelium of a papilloma and that of a papillary carcinoma. However, if myoepithelium can be demonstrated in the infarcted tissue, the tumor is more likely to be a papilloma than a papillary carcinoma. Cytologic atypia is commonly found in the partially degenerated epithelium of a papilloma in the vicinity of infarcts. The atypia is usually manifested by nuclear hyperchromasia and pleomorphism. These cytologic abnormalities may lead to an erroneous diagnosis of carcinoma in the fine-needle aspiration (
FNA) specimen from an infarcted papilloma
30,31 or in a needle core biopsy specimen.
Squamous metaplasia can occur in the epithelium of a papilloma (
Fig. 5.16). It is more likely to be found when there is infarction and probably represents a reactive or reparative process.
32 Rarely, squamous metaplasia constitutes a conspicuous component of the papilloma or of the epithelium lining the cystic portion of the lesion. Extension of squamous metaplasia to the epithelium of adjacent ducts is an uncommon finding.
33 Entrapped metaplastic epithelium in the stromal reaction may simulate metaplastic or squamous carcinoma, and in some instances the distinction between these processes is very difficult.
32 Electron microscopy and immunohistochemistry (
IHC) suggest that some examples of squamous metaplasia derive from myoepithelial cells.
34 Sebaceous metaplasia of luminal cells of a papilloma has also been reported.
35Intraductal papillomas can be recognized by frozen section (
FS), but in most circumstances, it is preferable to rely upon paraffin sections for the diagnosis of papillary tumors. It is sometimes difficult to prepare satisfactory FSs from these fragile lesions or to identify foci of carcinoma that have developed in a papilloma.
Cytology
The diagnosis of an intraductal papilloma may be suggested by the cytologic findings in an
FNA specimen.
36,37 The smear typically has cohesive three-dimensional groups of cytologically benign-appearing cells accompanied by variable amounts of stromal cells, apocrine cells, inflammatory cells, and histiocytes. Two diagnostic problems can arise in this setting.
First, a significant number of lesions classified as papillary on the basis of findings in needle aspiration specimens prove not to exhibit a papillary architecture when excised. Of the 70 cases studied by Simsir et al.
38 in which needle aspiration specimens were classified as papillary, only 31 (44%) showed a papillary architecture when examined histologically. FAs, fibrocystic changes (FCCs), a phyllodes tumor, and carcinomas accounted for the remaining 39 cases. Although usually correctly classified as benign, FAs and FCCs account for the most common discrepancies. Apocrine cells, foamy histiocytes, and bipolar nuclei associated with myoepithelial cells are characteristic of a papilloma. Although one can also observe these cells in aspirates from FAs, the epithelium from the latter lesion tends to be distributed in flat sheets rather than in three-dimensional groups, and it usually has a honeycomb appearance. Furthermore, the epithelial groups from FAs typically lack fibrovascular stroma, and one usually observes fragments of stroma devoid of blood vessels and unassociated with epithelial cells. Most aspirates of FCCs appear less cellular than those from papillomas, and they do not contain branched, three-dimensional cell clusters supported by fibrovascular stroma. These generalizations notwithstanding, many smears do not demonstrate the findings sufficiently clearly that one can make a secure diagnosis of papilloma. Even in retrospect, Simsir et al.
38 could not distinguish the smears of three FAs from those of papillomas. Mak and Field
39 recounted similar observations. The authors studied 56 cases in which smears were interpreted as papillomas. The excision specimens revealed papillomas in 42 (75%) of the cases yielding a
PPV of 0.74. FAs, radial scars, and other benign proliferative lesions accounted for 10 discrepancies, atypical ductal hyperplasia (
ADH) 2 discrepancies, and tubular carcinomas the remaining two discordant cases.
Second, the cytologic detection of malignancy in a papilloma and the recognition of a papillary carcinoma often prove especially difficult. For example, in the study of
FNA specimens of papillary lesions by Masood et al.,
40 3 of 21 tumors interpreted as benign papillary lesions proved to be micropapillary ductal carcinoma
in situ (
DCIS) upon study of an excision specimen. Immunostaining may help in the diagnosis of
FNA specimens of papillary breast tumors. Chang et al.
41 reported that the percentage of Ki67-positive cells was significantly higher in papillary carcinomas (21.0 ± 19.23%) than in papillomas (6.23 ± 7.25%). These investigators did not find cyclin-D1 reactivity to be a useful differential feature. Staining of cell-block preparations for calponin to reveal myoepithelial cells can provide diagnostic information in certain cases.
42 For further discussion, see
Chapter 14.
Core Biopsy
The diagnosis of papillary tumors of the breast by needle core biopsy has been the subject of extensive investigation, and the literature contains more than 40 reports dealing with this topic. Publications by Skandarajah et al.,
43 Bernik et al.,
44 Jung et al.,
45 and Brennan et al.
46 tabulate data from many investigations, and
Table 5.1 lists findings from those comprising more than 50 cases.
2,43,45,47,48,49,50,51,52,53,54 Interpretation of this body of information is hampered by several factors. First, most studies represent retrospective analyses of cases in which a core biopsy reportedly demonstrated a papilloma. In the early studies, only a few patients underwent excisions, so selection bias may have influenced the results. Second, the designs of the studies have varied widely. Certain ones included retrospective review of histological or radiologic studies, whereas others did not. The histologic classification of the papillary lesions differed, the expertise of the radiologists and pathologists varied, and most studies do not describe in detail the extent of the study of the core biopsy specimens. Statements about the concordance of the radiologic studies and the pathology studies were not included in every report. Finally, during the more than two decades spanned by these studies, the practices of both breast imaging and breast pathology have changed in ways such as the use of vacuum-assisted biopsy methods and immunohistochemical staining, and these advances could have influenced the findings of the studies.
Despite these significant differences in design, a coherent picture has emerged, and two generalizations seem established. First, an excision is usually indicated when a core biopsy specimen of a papillary tumor contains atypical ductal or lobular cells (
ADH, atypical lobular hyperplasia [
ALH], or lobular carcinoma
in situ [
LCIS]). Between one-third and one-half of such patients will prove to have a carcinoma in an excision specimen. This conclusion mirrors the approach advocated for atypical epithelial proliferations detected in other histologic settings. Second, patients in whom a core biopsy specimen discloses a benign papilloma have a significant likelihood of harboring an atypical epithelial proliferation in an excision specimen. The literature contains data from more than 2,000 core biopsy specimens showing only benign papillomas. Subsequent excision specimens demonstrated benign findings in approximately 80%, atypical ductal or lobular cells (
ADH,
ALH, or
LCIS) in approximately 10%, and
DCIS or invasive carcinoma in approximately 10%.
Researchers have attempted to improve the predictive values of the findings present in core biopsy specimens demonstrating benign papillomas by incorporating ancillary clinical, radiologic, or histologic features in their analysis. Scattered reports have identified findings that seem to indicate an increased risk for the presence of atypical cells in an excision specimen; however, other investigations have not borne out the predictive value of these findings in most instances. The literature does suggest that two features may identify patients at elevated risk for atypia: age and size of the lesion. Arora et al.
55 observed an increasing likelihood of an upgrade to malignancy with the increasing age of the patient, and five other studies found that older patients are more likely to have atypical cells discovered on excision.
46,53,56,57,58 This association notwithstanding, investigators have not discovered an age threshold below which an excision would seem unnecessary.
The size of a papilloma may also influence the likelihood of discovering atypical cells in an excision specimen. In the study of Jung et al.,
45 the presence of a palpable mass indicated an increased likelihood of the detection of carcinoma and so did the presence of a mass on the mammogram. Among studies examining imaging features, two reported that the presence of a mass greater than 1.5 cm detected by sonography
47 or by either sonography or mammography
51 indicated an increased likelihood of carcinoma in a subsequent sample. Another investigation
50 observed that benign papillomas associated with atypia in excision specimens were larger than those that were not. Three other studies did not confirm the predictive nature of the size of the lesion.
49,57,58Additional features such as clinical information, radiologic findings, details of the biopsy procedure, and the experience of the radiologists have not improved the predictive values consistently. Many authors stress the need for careful correlation of the radiologic and pathologic findings and point out that lack of correlation accounts for many discrepancies. Nevertheless, in the study by Bernik et al.,
44 9 of 17 benign papillomas with concordant radiologic studies proved to have
ADH in the excision specimen, and 2 of the 17 contained carcinomas. Other authors report the same experience.
Pathologists have searched for way to improve their diagnostic abilities, and both the use of immunohistochemical stains and the expertise of pathologists have come under study. Shah et al.
59 found that the use of immunohistochemical staining for calponin, CK5/6, and p63 allowed pathologists to recognize foci of
ADH associated with papillomas. This technique improved the accuracy of all four participating pathologists to the point that they concluded that papillomas lacking atypia “do not require excision in the absence of suspicious clinical/radiological findings.” Tse et al.
60 employed staining for estrogen receptor (
ER), CK14, and p63 in an attempt to resolve discrepancies encountered in 15 core biopsy specimens. Although the use of these stains reduced the rate of discordance by 69%, the staining results did not eliminate either false-positive or false-negative cases.
The expertise of the pathologist influences the correlation between findings of core biopsy and excision specimens, but even experienced breast pathologists cannot exclude the presence of atypical cells in an excision specimen. In the study of Jakate et al.,
50 diagnoses made by pathologists with fellowship training in breast pathology on core biopsy specimens were less likely to differ from those of the subsequent excision specimen than were diagnoses made by general pathologists; nevertheless, a substantial number of discrepancies remained. An “upgrade” rate of 26.3% was observed for diagnoses made by general pathologists, whereas a rate of 16.3% was observed for breast pathologists. Of the 86 cases in which breast pathologists made a diagnosis of benign papilloma on a core biopsy specimen, 10 excision specimens displayed atypia and 3 showed malignancy.
Several investigations include patients who did not undergo an excision following the diagnosis of benign papilloma on a core biopsy. The authors reported that 7 of the 437 patients (1.6%) included in these studies developed carcinoma. Followup periods ranged from 2 to 5 years, and the nature of the follow-up varied. These variations, among many other aspects of the studies, make it impossible to draw secure conclusions about the safety of careful clinical surveillance as an alternative to excision of benign papillomas diagnosed by means of a core biopsy. A conservative approach may prove safe, but investigators have not formulated the criteria to identify either the appropriate patients or the details of the program of surveillance.
Data from the foregoing studies do not provide definitive guidelines for the management of a papilloma without atypia diagnosed by needle core biopsy. A decision as to whether surgical excision should be performed will be influenced by factors such as the size of the lesion, evidence of residual tumor following the biopsy, the ease of mammographic follow-up, family history of breast cancer, and patient concerns. Among women recommended for follow-up without an initial surgical excision, some will require surgical biopsy at a later date. In a series studied by Sexton et al.,
61 59 of 78 patients (i.e., 75%) with a papilloma diagnosed by needle core biopsy did not undergo surgical biopsy and were followed for 3 to 5 years. Subsequent interval mammographic changes necessitated surgical excision in 10 of the 59 (17%), and 2 (3%) had a subsequent needle core biopsy. All subsequent biopsies were reportedly “benign.” This study suggests that up to 20% of patients enrolled in follow-up after a needle core biopsy diagnosis of papilloma will undergo another biopsy within 5 years of the initial procedure. The long-term risk for the development of carcinoma at the site of an incompletely excised papilloma that was sampled by core biopsy has not been determined.