Pancreaticoduodenectomy: Minimally Invasive Resection
Song Cheol Kim
Ki Byung Song
DEFINITION
Pancreaticoduodenectomy (PD) is defined as the resection of the pancreatic head, duodenum, bile duct, and gallbladder, with or without the distal stomach (pylorus preserving), for benign or malignant periampullary diseases.
Gagner and Pomp first introduced laparoscopic pyrolus preserving pancreatoduodenectomy (LPPPD) in an advanced laparoscopic surgical trial in 1994. Subsequently, there has been technical progress in laparoscopic pancreatoduodenectomy (LPD) that includes hand-assisted LPD as well as laparoscopic-assisted and robot-assisted PD.
DIFFERENTIAL DIAGNOSIS
Pancreatic cancer
Distal bile duct cancer
Ampullary cancer
Duodenal cancer
Pancreatic neuroendocrine neoplasms
Others
PATIENT HISTORY AND PHYSICAL FINDINGS
Not all patients are suitable candidates for LPD. Table 1 shows the currently accepted contraindications for LPD. LPD with portal vein (PV) resections and reconstructions has been reported. With increasing experience, LPD can be attempted in obese patients.
A history of extensive, previous abdominal surgery or pancreatitis may preclude successful completion of LPD. Hence, a detailed patient history and medical record review is vital to minimize the rate of conversion.
A high body mass index (BMI) should be considered a significant factor for morbidity, especially during the learning period. Patients with a high BMI usually have a large amount of parietal and visceral fat, which makes identification of the detailed structures of the inner abdominal organ challenging. Exposure of the third and fourth portions of the duodenum is particularly challenging in the obese patient. Furthermore, the fragile nature of a fatty pancreas leads to difficulties in manipulating or suturing the organ during anastomosis.
Table 1: Current Contraindications for Laparoscopic Pancreaticoduodenectomy | ||
|---|---|---|
|
IMAGING AND OTHER DIAGNOSTIC STUDIES
Patients require abdominal or abdominopelvic computed tomography (CT) or magnetic resonance imaging for diagnosis and surgical planning. Multidetector CT (MDCT) is a useful diagnostic tool for detecting the vascular involvement of the tumor, including the PV, the superior mesenteric vein (SMV) or artery, and the celiac axis. MDCT has been reported to have a sensitivity of 85% to 95% and a specificity of 95% for pancreatic cancer.
The advent of MDCT has eliminated the need for routine magnetic resonance imaging. Heavily weighted T2 imaging sequences in magnetic resonance cholangiopancreatography can depict the pancreatic duct, biliary tree, liver, and vascular structures. Magnetic resonance cholangiopancreatography can replace endoscopic retrograde cholangiopancreatography for imaging of pancreatic and biliary lesions, thereby avoiding endoscopic retrograde cholangiopancreatographyrelated complications such as bleeding, perforation, and pancreatitis.
Endoscopic ultrasound (EUS) with a high-frequency probe produces a high-resolution image of the pancreas and its surrounding structures; a small pancreatic mass or cancer can be detected with a sensitivity of 91% to 99% and a specificity of 100%. EUS fine needle aspiration (EUS-FNA) is useful for the diagnosis of an indeterminate pancreatic cystic lesion or when a clinical diagnosis prior to operation is clinically indicated. However, EUS-FNA is not mandatory in operable patients who have been diagnosed with a resectable surgical pancreatic mass by conventional CT and who have clinical signs compatible with a malignant diagnosis. Surgeons should also be mindful of possible risk factors and complications that are associated with an extended surgical time, a possibility that may arise during LPD. (See the guidelines of the Society of American Gastrointestinal Endoscopic Surgeons.)
SURGICAL MANAGEMENT
Preoperative Planning
Preoperative palliation of obstructive jaundice may be appropriate for jaundiced patients but such procedures are associated with an increased risk of operative complications.
In the case of colon or mesocolon invasion, a bowel prep should be considered before the operation.
Prophylactic antibiotics and chemical and mechanical venous thromboembolism prophylaxis are warranted.
TECHNIQUES
POSITIONING
The patient is placed in the supine position with pads underneath the right side of the back to elevate the right side of the abdomen. Alternatively, a split-leg positioning can be used for LPPPD. In this position, the operator stands between the legs of the patient and the assistants stand on either side of the patient.
A nasogastric tube and Foley catheter are inserted to decompress the stomach and bladder, respectively.
Reverse Trendelenburg (10 degrees to 30 degrees) position is used to expose the operative field.
Two monitors are placed at the sides of the operator and first assistant. The primary surgeon and second assistant, who holds the laparoscope, stand to the right of the patient, and the first assistant and the scrub nurse are positioned to the left of the patient. Intravenous patientcontrolled analgesia systems are used to control the pain after the operation.
TROCAR PLACEMENT
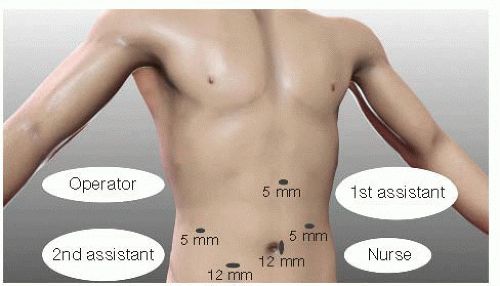
Open technique is used to establish the pneumoperitoneum through a 12-mm trocar on the umbilicus.
Thirty-degree angled vision scope is used for visualization of the deep portion. The trocar locations are shown in FIG 1.
An additional three or four trocars are placed under direct vision. Two or three 5-mm trocars (one on the right flank for the left hand of the surgeon and one or two on the left flank for surgical assistance, if necessary) and two 12-mm trocars (one for the laparoscope and one on the umbilicus for the right hand of the surgeon) are employed.
Abdominal pressure is maintained at 12 mmHg using carbon dioxide (CO2) gas insufflation. Keeping the abdominal inflation pressure low, not more than 12 mmHg, is important to minimize accumulation of CO2 gas; the LPD procedure can routinely take more than 6 hours.
IDENTIFICATION OF THE PORTAL VEIN AND DIVISION OF THE DUODENUM OR STOMACH
The entire abdomen is examined for any abnormalities or metastasis. The entire hepatic and peritoneal surfaces should be inspected. Intraoperative ultrasound may be used for further examination.
The gastrocolic omentum is dissected to allow entry into the lesser sac (FIG 2).
The PV is identified at the inferior border of the pancreas by distally following the gastroepiploic vein (GEV) to its insertion into the SMV. The GEV is clipped and divided as it inserts into the SMV (FIG 3). The GEV often drains into the middle colic vein, and in this circumstance, efforts should be made to preserve the middle colic vein.
The anterior aspect of the retropancreatic segment of the PV/SMV is dissected, creating a tunnel, and potential invasion of tumor into the PV is assessed (FIG 4).
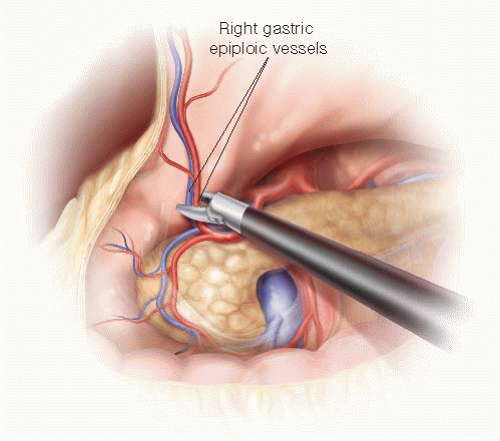
The gastrohepatic omentum is opened to expose the hepatic artery coursing cephalad to the pancreas. The right
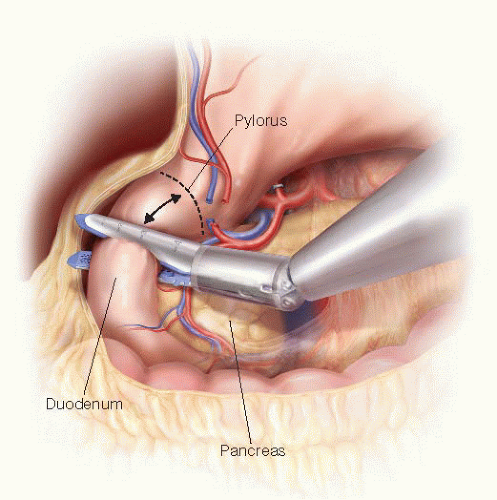
gastric artery is ligated using a metal clip or Harmonic scalpel. After dividing the branches of the right gastroepiploic vessels along the duodenum, the duodenum is divided 2 cm distal to the pylorus using an endoscopic linear stapler (FIG 5). Alternatively, resection of the gastric antrum can be performed according to surgeon preference or when there is concern for obtaining an adequate margin. The stomach is placed in the upper region of the abdominal cavity.
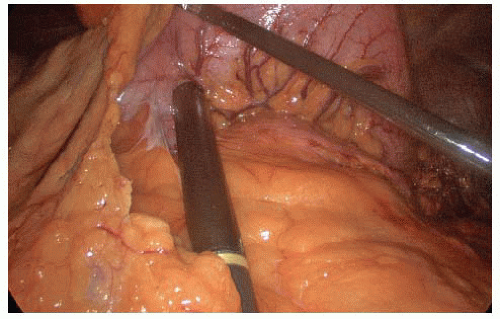
FIG 2 • Laparoscopic ultrasound. Laparoscopic intraoperative ultrasound probe is inserted into the 12-mm trocar, and ultrasonography is performed for identifying the location of the lesion.
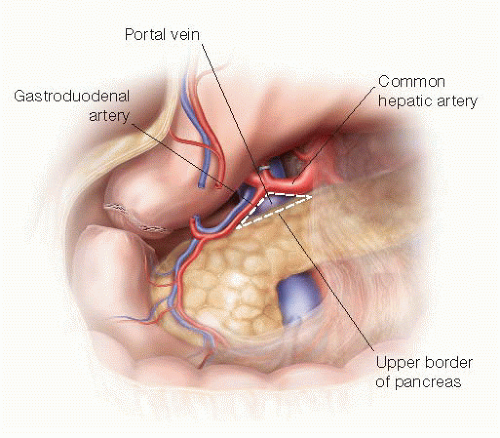
The upper border of the pancreas is dissected to establish “Kim’s triangle,” formed by the common hepatic artery, upper border of the pancreas neck, and gastroduodenal artery (GDA) (FIG 6). The GDA is ligated at its origin and then divided with a vascular staple load. The author recommends marking this vessel as well using a clip.
The PV tunnel is completed and gentle upward traction of the isolated pancreas is applied using an umbilical tape in preparation for division of the pancreas.
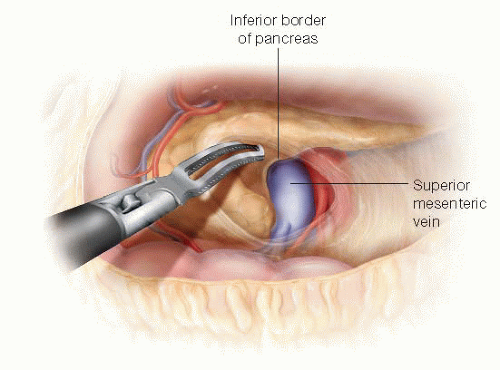 FIG 4 • Identification of PV and SMV. The SMV is identified at the inferior border of the pancreas and dissected up to the retropancreatic PV. |
MOBILIZATION OF THE RIGHT COLON AND DUODENUM AND IDENTIFICATION OF THE SUPERIOR MESENTERIC VEIN
The peritoneum of the hepatic flexure of the right colon is incised. The right colon is mobilized downward and to the left side of the patient to fully expose the second and third portions of the duodenum. The dissection between the mesocolon and the duodenum/pancreatic head proceeds along the avascular surgical plane and is facilitated by the first assistant pulling the mesentery of the right colon toward the patient’s left lower quadrant (FIG 7). Any remaining branches of right gastroepiploic vessels are divided (FIG 8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree