Pancreaticoduodenectomy for Chronic Pancreatitis
Keith D. Lillemoe
Chad G. Ball
Achieving effective treatment for patients with chronic pancreatitis is one of the most difficult challenges in all of surgery. Irrespective of etiology, it is also a disease that displays a notably worse actuarial survival compared to age-matched controls. The most significant complications associated with chronic pancreatitis are pain (50% at 15 years), diabetes (75% at 15 years), and exocrine insufficiency (80% at 15 years). Operative procedures to treat chronic pancreatitis are classically indicated for specific symptoms including: (a) intractable pain (60%), (b) biliary obstruction (<5%), (c) duodenal stenosis (<5%), and (d) the need to exclude a diagnosis of malignancy (30%). Less common indications include: (a) bleeding, (b) complicated pseudocyst, and (c) sinistral hypertension. In summary, chronic pancreatitis becomes an operative disease only when there is a need to treat complications of the underlying pathology.
Although intractable pain represents the most common indication for operative intervention, the precise etiology of this discomfort remains unclear. Popular theories defining the origin of pain include (a) ductal hypertension, (b) parenchymal hypertension, and (c) neural inflammation among the primary afferent neurons. The neuronal theory holds particular weight given the dramatic increase in both the number and the size of nerves found within the gland in the patients suffering from chronic pancreatitis.
Despite the above-mentioned operative indications, it is crucial to recognize that chronic pancreatitis is often a direct consequence of complex and long-standing social factors. More specifically, the surgeon must address the overall psychology of the patients, their ability to follow a treatment plan that includes abstinence from alcohol, as well as a commitment to engage in physician-guided weaning of narcotics after the operative procedure. Without this thorough evaluation on the part of the surgeon, and commitment on the part of the patient, a significant percentage of patients who undergo operative procedures will fail to achieve a successful therapy. This philosophy can be extended to all causes of chronic pancreatitis in that the underlying etiology must always be eliminated. As an example, if a patient continues to consume alcohol following an operation that results in insulin dependent diabetes, the chances of life-threatening complications are substantial.
The technical armamentarium of the pancreatic surgeon currently includes both duodenal-preserving and drainage procedures (Frey, Beger, Peustow, Duval) and formal resection (distal pancreatectomy, pancreaticoduodenectomy, total pancreatectomy) procedures. The selection of one technique over another should be based on the primary location of disease (head versus body/tail), the size of the pancreatic duct, and the presence of a stricture of the bile duct. The use of these various operative interventions in the modern era also reflects a shift in paradigm from parenchymal-based (1950s to 1980s), to duct size-based (1980s to 1990s), to resection and drainage-based (current) philosophies. In cases selected for a pancreaticoduodenectomy, the vast majority of the pancreatic inflammatory process should be located in the head of the gland. This view is particularly important for those who believe that the epicenter of inflammation, and therefore the “pacemaker” of intractable pain, is located in the head of the gland. Additional advantages of this procedure include concurrent decompression of a bile duct stenosis as well as the ability to safely perform a reconstruction in the presence of a small pancreatic duct. Finally, pancreaticoduodenectomy is the appropriate “cancer operation” should consideration of the malignancy be part of the differential diagnosis.
In summary, the patients with a diagnosis of chronic pancreatitis who are eligible for a pancreaticoduodenectomy represent a highly selected subgroup. They display head-predominant disease in the setting of intractable pain, and not infrequently have a concurrent biliary duct stricture or duodenal stenosis. These patients do not typically overlap with those who would benefit from a pancreas-sparing procedure or distal pancreatectomy. If these prerequisites are strictly adhered to, the patients will have an excellent chance at good relief of their disabling pain.
In addition to a thorough history and assessment of a patient’s social risk factors, preoperative evaluation is also heavily dependent on the use of modern diagnostic imaging. Options include, but are not limited to, contrast-enhanced computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), endoscopic ultrasound (EUS), and endoscopic retrograde cholangiopancreatography (ERCP). Each technique offers varying degrees of information on both the pancreatic duct and the gland itself. The appropriate selection of these diagnostic techniques is crucial in both determining whether the extent of disease is appropriate for surgical management and also in guiding the choice of the most appropriate operative procedure for each individual patient based on ductal anatomy and gland morphology. A triple-phase intravenous contrast helical CT (fine-cut pancreas protocol) is typically the initial noninvasive imaging test for pancreatic disease, having largely supplanted ultrasonography in this context. Helical CT displays the anatomy of the pancreas gland in considerable detail. It can also easily define pancreatic calcifications, inflammation, necrosis, and masses. MRCP can be quite useful for noninvasively defining the anatomy and pathology of both the bile ducts and the pancreatic duct. MRCP can be especially helpful in the cases where the ampulla of Vater is not accessible (i.e., the patients who have previously undergone Roux-en-Y or Billroth II reconstructions). Specific EUS criteria exist to define parenchymal (echogenic strands, hyperechoic foci, calcifications, lobular contour, cysts) and ductal (wall hyperechogenicity, irregular duct wall, visible side branches, strictures, stones) disease, but technique is highly operator dependent. Finally, ERCP provides direct imaging of the pancreatic and bile ducts and is the gold standard for diagnosing chronic pancreatitis. ERCP also allows therapeutic stenting of biliary and pancreatic duct strictures both as temporary measures for short-term management or definitive treatment.
Resection of the pancreatic head in the setting of chronic pancreatitis can be accomplished by means of either a pylorus-preserving pancreaticoduodenectomy (PPPD; Fig. 1) or the classic Whipple resection (including an antrectomy). Multiple randomized trials have failed to show any significant differences between the two procedures in terms of either relative ease of performance or short- or long-term outcome in the patients with pancreatic cancer. As a result, the choice between procedures for either chronic pancreatitis or cancer is usually made on the basis of individual surgeons’ preferences. Our preference is typically the pylorus-preserving modification.
Preoperative mechanical bowel preparations are optional. Antibiotics that prevent enteric bacteria are administered at induction of anesthesia. Antibiotic consideration must also account for the patients who have had biliary or pancreatic duct stents.
Surgical Technique—Pylorus-Preserving Pancreaticoduodenectomy (Pppd)
The peritoneal cavity is entered through an upper midline or a bilateral subcostal incision. The round ligament is divided at the liver and the portion between the liver and the umbilicus is excised. A fixed abdominal wall retractor system of the surgeon’s choice is engaged after a wound protector is placed. Especially in the cases where a malignancy must be ruled out, the liver, the omentum, and the peritoneal surfaces are inspected and palpated. Any suspicious lesions are biopsied and submitted for frozen-section analysis.
Resection of the Specimen
Operative assessment begins with a wide Kocher maneuver and mobilization of the duodenum and the head of the pancreas from the underlying inferior vena cava and aorta. When the duodenum and head of the pancreas have been mobilized sufficiently, a hand is placed under the duodenum and the head of the pancreas to palpate the local anatomy. The lesser sac is then opened widely by incising the lesser omentum and by removing the entire greater omentum off the transverse colon. Every attempt is made to preserve the greater omentum. The hepatic flexure of the colon is also mobilized and retracted downward out of the operative field early in the operative procedure.
Our preference is to dissect the SMV and portal vein as an initial step. The inflammatory response associated with chronic pancreatitis can be considerable making this dissection somewhat tedious, therefore early control is important if bleeding is encountered at any point in the case. Identification of the portal vein is greatly simplified if the common hepatic duct is divided early in the dissection. Once the hepatic duct has been divided, the anterior surface of the portal vein is easily and quickly identified. The lymph node tissue lateral to the hepatic duct and the portal vein should be dissected off the structures to be included in the surgical specimen. This is somewhat less important in cases of chronic pancreatitis where a malignancy is not the primary concern. It must be remembered that important variations in the hepatic arterial anatomy, including a replaced right hepatic artery, may be encountered during this dissection. If the appropriate plane is found along the anterior surface of the portal vein, it is often possible to pass the index finger of the left hand on top of the vessel posterior to the first portion of the duodenum and the neck of the pancreas (because usually there are no veins joining the anterior surface of the portal vein). If this maneuver proves difficult, which is not uncommon in cases of chronic pancreatitis because of adherence of the fibrotic pancreas gland to the SMV and portal vein, the gastroduodenal artery should be identified where it originates from the common hepatic artery. Once adequate dissection has been carried out, the artery should first be clamped with a nonoccluding vascular clamp, then, if the hepatic artery pulse is preserved, divided and ligated with 2-0 silk ties. (The initial clamping of the gastroduodenal artery with a vascular clamp confirms that the arterial supply to the liver will not be interrupted should either variations in hepatic arterial anatomy or important collateral circulation be present in the face of celiac artery stenosis.) After the artery has been divided and ligated, an additional ligature of 3-0 polypropylene should be placed on the proximal stump. Division of the gastroduodenal artery unroofs part of the tunnel through which the index finger is slipped, thereby greatly facilitating the separation of the portal vein from the posterior aspect of
the first portion of the duodenum and the neck of the pancreas.
the first portion of the duodenum and the neck of the pancreas.
Once the anterior surface of the portal vein has been dissected posterior to the neck of the pancreas, the next step is to identify the SMV and dissect its anterior surface. This is most easily accomplished by extending the Kocher maneuver past the second portion of the duodenum to include the third and fourth portions. During this extensive kocherization, the first structure encountered anterior to the third portion of the duodenum is the SMV. The anterior surface of the vein can then be cleaned and dissected under direct vision by retracting the neck of the pancreas anteriorly. This dissection is continued until it connects to the portal vein dissection from above.
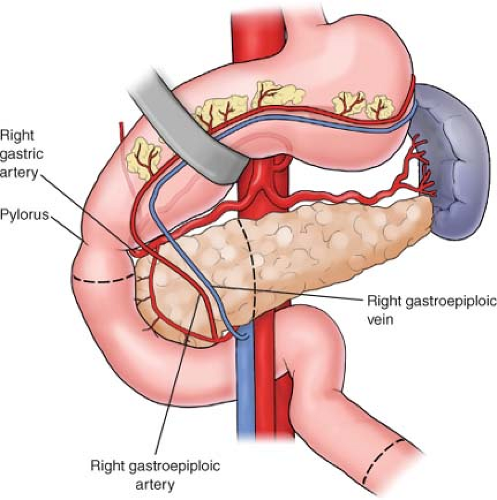
In a PPPD, the duodenum is first mobilized and divided <2 cm distal to the pylorus with a gastrointestinal anastomosis (GIA) stapler. The posterior surface of the proximal first portion of the duodenum is dissected until the lesser sac is entered. At this point, the soft tissue attachments from the inferior border of the duodenum to the inferior border of the pancreas are divided. The right gastroepiploic vessels, which can be sizable, are clamped, divided, and ligated. In a similar fashion, the soft tissue areolar attachments found superiorly are divided with the electrocautery. The right gastric artery, which originates from the common hepatic artery, is also ligated.
In a classic Whipple procedure, an antrectomy is performed. The right gastroepiploic arcade and the right gastric vessels are divided to permit mobilization of the antrum. The stomach is then divided with a GIA stapler, usually at the level of the incisura. At this point, if the gastroduodenal artery was not divided earlier, it is identified, divided, and ligated as described (see above). During this step, particular care must be taken to ensure that the lumen of the common hepatic artery is not encroached on by one of the proximal ties.
The neck of the pancreas is then divided with the electrocautery, with care taken not to injure the underlying SMV and portal vein. The head of the gland is then reflected to the right and head and the uncinate process of the pancreas is dissected away from the portal vein and SMV. In the patients with chronic pancreatitis, this dissection can again be difficult because of adherence between the fibrotic gland and the SMV. Great caution must be taken for all dissection around the SMV and portal vein to prevent a major vascular injury and subsequent hemorrhage. If a replaced right hepatic artery is present, its origin from the superior mesenteric artery (SMA) will be encountered at this point and must be preserved. The uncinate process can then be divided between clamps flush with the SMA, then ligated with 2-0 silk ties; alternatively, either an energy-based vessel-sealing system or ultrasonic shears may be used. The SMA is completely exposed during this dissection, which proceeds from cephalad to caudad, as shown in Figure 2. Unlike PPPD for cancer, the extent of this dissection, and therefore the inclusion of the lymphatic tissue adjacent to the SMA, is less critical in the patients with chronic pancreatitis. As a rule, there are two large veins joining the SMV inferiorly that must be dissected free, doubly ligated, and divided.
Once the uncinate process has been completely divided, the specimen is attached only by the third portion of the duodenum. At this point, the upper abdomen is copiously irrigated with an antibiotic solution and packed. The transverse colon, along with the greater omentum, is reflected cephalad. The proximal jejunum and the ligament of Treitz, along with the fourth portion of the duodenum, are dissected free, and the dissection is continued until it meets the right-side upper abdominal dissection. At a convenient point where there is a wide vascular arcade, the proximal jejunum is divided with a GIA stapler <10 to 12 cm distal to the ligament of Treitz. The proximal jejunum is then grasped with a Babcock clamp and retracted cephalad. The mesentery to the proximal jejunum is divided between clamps and ligated with 2-0 silk suture (or divided with a vessel-sealing energy device). Alternatively, dividing the jejunum early, before dividing the uncinate process, can help with retraction and exposure especially in the face of local inflammation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree