Pancreatic Pseudocyst: Laparoscopic or Endoscopic Versus Conventional Surgery
Gary C. Vitale
Brian R. Davis
In the past 15 years, the management of pancreatic pseudocysts, abscesses, and necrosis has undergone radical changes with the advent of new endoscopic and laparoscopic drainage techniques. These approaches are applicable to both acute fluid collections and chronic pseudocysts associated with pancreatic necrosis and abscess.
The different types of peripancreatic fluid collections have been defined by the Atlanta Classification described by Bradley (1993). According to this classification, peripancreatic fluid collections can be categorized based on computed tomography (CT) findings by the presence of a collection, relation of the collection to the pancreas, content, shape, mass effect, loculations of gas bubbles, and air fluid levels. Acute fluid collections occur within the first 4 weeks following an attack of acute pancreatitis and may resolve spontaneously. Pancreatic necrosis is defined as focal or diffuse areas of nonviable pancreatic parenchyma greater than 3 cm in diameter or involving more than 30% of the area of the pancreas. Pancreatic pseudocysts are defined as having a fibrous wall of granulation tissue and result from fluid collections persisting more than 4 weeks after the onset of an episode of acute or chronic pancreatitis. According to Jacobson (2005), pancreatic necrosis (>25%) is a risk factor for pseudocyst development, and these fluid collections persist and form pseudocysts in 5% to 15% of patients with acute pancreatitis and up to 40% of patients with chronic pancreatitis. Pancreatic abscesses are defined as an intra-abdominal collection of pus containing little or no pancreatic necrosis occurring 4 weeks after onset of the pancreatitis. Acute fluid collections that form in the presence of pancreatic necrosis have been classified by terms such as walled-off pancreatic necrosis (WOPN), organized necrosis, and necroma. Surgical laparotomy drainage has previously been the procedure of choice for pancreatic abscesses and necrosis, but endoscopic and laparoscopic techniques have become routine whether treating pseudocysts or substantial pancreatic necrosis.
Choice of Treatment for Pancreatic Pseudocyst
Conservative Versus Surgical Treatment
Most patients with pancreatic pseudocysts can be managed conservatively if presenting symptoms are well controlled. Up to 40% of acute pseudocysts are reabsorbed after pancreatitis resolves. When pancreatic pseudocysts are large enough to cause symptoms (6 cm or greater) the success of conservative therapy is low (between 3%
and 39%) according to Bradley (1979). More recent studies by Vitas and Sarr (1992) have demonstrated that approximately half of acute pancreatic pseudocysts remain asymptomatic regardless of size or duration.
and 39%) according to Bradley (1979). More recent studies by Vitas and Sarr (1992) have demonstrated that approximately half of acute pancreatic pseudocysts remain asymptomatic regardless of size or duration.
Pseudocysts greater than 6 cm in diameter that show no evidence of spontaneous resolution should be considered for therapeutic intervention. Observation for cyst resolution should occur for 6 weeks following the incidence of pancreatitis to allow for cyst maturation. Endoscopic retrograde cholangiopancreatography (ERCP) should be performed for pseudocysts resulting from blunt abdominal trauma since there is a high incidence of pancreatic duct disruption in these cases. If ERCP demonstrates a major pancreatic duct disruption, consideration should be given to a pancreatic resection. A series followed by Lawrence (2008) demonstrated that out of 29 patients with pancreatic pseudocysts and complete duct disruption, 50% had recurrent fluid collections following surgical and endoscopic pseudocyst drainage and required long-term follow-up for symptoms related to this disorder.
Pseudocysts that are asymptomatic and less than 6 cm in size require no treatment, and these patients can be safely followed by CT at 3- to 6-month intervals. Pseudocysts associated with chronic pancreatitis are unlikely to resolve spontaneously. Indications for pseudocyst drainage include the presence of symptoms and enlargement of the pseudocyst. Complications from pancreatic pseudocysts include infection, hemorrhage, rupture, obstruction, and malignancy. Most commonly patients present for surgical therapy complaining of persistent abdominal pain, gastric outlet obstruction, jaundice, and dyspepsia with weight loss.
Patients that have multiple medical comorbidities and are considered a poor risk for surgical intervention should be treated initially with percutaneous CT-guided catheter drainage. Percutaneous drainage is utilized for the treatment of immature and infected pseudocysts. This technique can be complicated by formation of an external pancreatic fistula, secondary infection of the pseudocyst, as well as a high recurrence rate. Contraindications to percutaneous drainage include intracystic hemorrhage and pancreatic ascites. Heider (1999) reviewed the use of percutaneous catheter drainage in 66 patients, which demonstrated successful drainage in only 43% of patients with a 16% mortality rate and a 64% incidence of complications. Curry (2009) described an innovative percutaneous cyst-gastrostomy using interventional radiologic techniques to place transgastric stents in a series of 12 patients that demonstrated a 58% success rate and a 17% complication rate. Secondary infection rates were 20% to 25% in the respective series that evaluated patients treated by interventional radiologists, as they were considered unfit for surgery secondary to medical comorbidities.
The most effective treatment of pancreatic pseudocysts involves internal drainage into the enteric lumen whether performed by endoscopic transmural versus transpapillary therapy, laparoscopic pseudocyst enterostomy, or standard laparotomy with cyst-enterostomy. Pancreatic resection is also indicated when there is severe pancreatic duct disruption or a suspicion of malignancy. The choice of which procedure is most appropriate depends on the condition of the patient; the size, number, and location of the pseudocysts; the presence or absence of communication of the pseudocyst with the pancreatic duct; the presence or absence of infection; and any suspicion of malignancy. Mature pancreatic pseudocysts should be first approached with endoscopic drainage if the practitioner is experienced in this procedure. Endoscopic pseudocyst drainage is less invasive, less expensive, and has a lower risk of creating an external pancreatic fistula than laparotomy or laparoscopic techniques. In the absence of an endoscopist experienced with pancreatic pseudocyst drainage, laparoscopic or open pancreatic pseudocyst-enterostomy is the procedure of choice.
Endoscopic Drainage
The evolution of endoscopic instruments and techniques is making this approach to pancreatic pseudocysts drainage more attractive, allowing for safe entry into the pseudocyst cavity as well as effective debridement of pancreatic necrosis. Given the low complication and mortality rates and the high rate of success for endoscopic drainage compared with surgery, surgical intervention should be reserved for select cases. The use of endoscopic ultrasound (EUS) to assist pseudocyst drainage further enhances the success of these procedures and reduces complication rates.
ERCP plays an important role in defining the anatomy of pancreatic pseudocysts. The index ERCP should demonstrate a complete pancreatogram. Evaluation of pseudocysts should also include a review of a high-quality CT scan. Endoscopic drainage should be the first preference for the treatment of mature pseudocysts, particularly those involving the pancreatic head. Endoscopic transpapillary drainage is effective for pseudocysts that communicate with the pancreatic duct while endoscopic transmural drainage offers good results in the management of suitably located pancreatic pseudocysts that complicate chronic pancreatitis. Endoscopic drainage is associated with higher rates of failure in infected pseudocysts and in the presence of pancreatic necrosis. Technically, endoscopic management of pancreatic pseudocysts is most effective when either there is communication with the pancreatic duct for transpapillary drainage or there is a visible indentation of the enteric lumen for a transmural approach. EUS can be utilized to detect the optimal location for pseudocyst puncture when there is no obvious indentation of the enteric lumen and to avoid hemorrhagic complications.
Endoscopic Techniques
Transpapillary Drainage
A side-viewing duodenoscope is passed through the oropharynx and the pylorus to the level of the ampulla of Vater. During scope insertion, the gastric wall is examined to evaluate for wall indentation suitable for transmural drainage. The endoscope is reduced into the short position by withdrawing the scope, and the ampulla is injected to opacify the biliary and pancreatic ductal systems. Biliary access is obtained by directing the sphincterotome superiorly and anteriorly in the ampulla toward the 11 o’clock direction. Sphincterotomy is first performed on the bile duct and then selectively on the pancreatic duct. Selective pancreatic sphincterotomy is accomplished by directing a guide wire with a hydrophilic tip inferiorly in the 3 o’clock position, posterior and to the right of where the biliary orifice is located. Pseudocyst demonstration on pancreatogram makes transpapillary drainage possible. Pancreatic duct sphincterotomy should be performed regardless of whether there is successful pseudocyst drainage. Decompression of the pancreatic duct including stenting can be helpful when there is direct communication with the pseudocyst. The transpapillary approach eliminates the risk of hemorrhage and retrogastric perforation associated with transmural pseudocyst drainage.
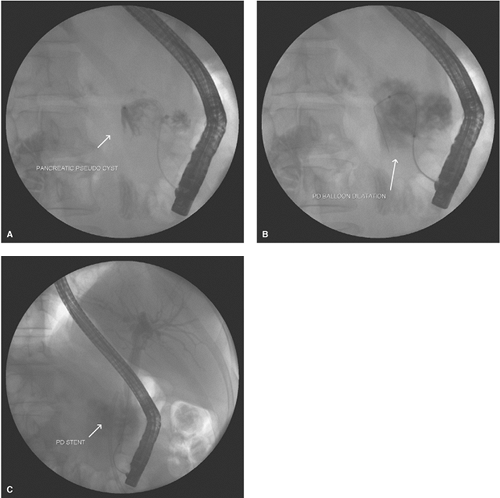
If the anatomy demonstrates pseudocyst opacification, an attempt is made to pass a guide wire through the ampulla and pancreatic duct into the cavity. A straight tip hydrophilic guide wire is attempted at first followed by an angled tip wire. A stent with appropriate length and a caliber of 7 French is then passed over the guide wire into the pseudocyst (Fig. 1A to C). The stent drains
the pseudocyst contents into the duodenum. When the anatomy demonstrates pancreatic duct disruption in communication with the cyst and a guide wire cannot be passed into the cyst cavity, a stent should be placed in the duct with its distal tip as close as possible to the origin of the pseudocyst. When the tail of the pancreas is not demonstrated on the pancreatogram, then there is complete duct disruption and the distal duct drains into the pseudocyst. If the tail section of the pancreatic duct can be visualized beyond the origin of the pseudocyst, passing a pancreatic stent into the disconnected duct may allow the duct disruption to heal and the cyst to resolve.
the pseudocyst contents into the duodenum. When the anatomy demonstrates pancreatic duct disruption in communication with the cyst and a guide wire cannot be passed into the cyst cavity, a stent should be placed in the duct with its distal tip as close as possible to the origin of the pseudocyst. When the tail of the pancreas is not demonstrated on the pancreatogram, then there is complete duct disruption and the distal duct drains into the pseudocyst. If the tail section of the pancreatic duct can be visualized beyond the origin of the pseudocyst, passing a pancreatic stent into the disconnected duct may allow the duct disruption to heal and the cyst to resolve.
The stent remains in the pancreatic duct until the pseudocyst resolves or significantly decreases in size as demonstrated by CT. Most patients demonstrate rapid pseudocyst resolution. Stent removal, reevaluation, and replacement should occur in 6 to 8 weeks. If the tail of the pancreas is not opacified at initial ERCP, it is likely that the distal duct is obstructed and may be feeding the pseudocyst. In these cases, concomitant transmural drainage may be necessary to adequately drain the tail if the cyst is enlarging. A magnetic resonance cholangiopancreatogram (MRCP) should be done in these cases to determine pancreatic tail duct anatomy to direct future therapeutic options.
Transmural Drainage
Upon examination of the gastric or duodenal lumen, those patients with pseudocyst indentation of the enteric lumen are candidates for transmural drainage by direct cyst-gastrostomy or duodenostomy. The cyst should indent the lumen enough to be recognized easily and the indentation should correspond with the cyst location on CT. Several techniques can be used for cyst-enterostomy. Our initial technique, at the University of Louisville, utilized a needle knife to open the indented area of the enteric lumen. A small area of 3 to 5 mm of mucosa is cauterized, and the needle knife is used to puncture the center of this area. Extrusion of cyst fluid is encountered when the cyst is entered, and a guide wire is then passed though the center of the needle knife into the cavity. This requires removal of the knife apparatus in a single lumen instrument, while the wire can be passed alongside the knife in double lumen instruments. A sphincterotome can be passed over the
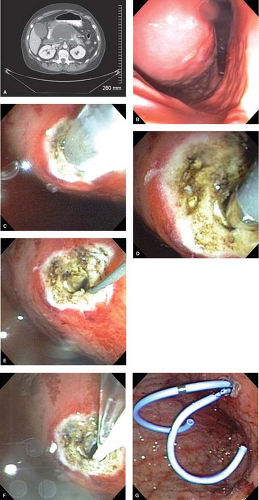
wire to enlarge the cyst-enterostomy. An opening of a minimum of 1 cm is preferable and is created by orienting the sphincterotome in multiple directions. A radial expansion balloon can be used to dilate the cyst-enterostomy in cases where there is bleeding from the mucosa. The pseudocyst is then drained with one to two 10 French stents, side by side (Fig. 2A to G). Double stenting is more effective than a single stent because the pseudocyst can drain alongside the stents as well as through them. Single stents can be encased by gastric mucosa and induce infection when their lumen becomes occluded. Since these stents remain in place long term, drainage alongside the stent may be more important than drainage through the stent, thus supporting the use of double stenting. The University of Louisville experience routinely dictates leaving stents in place for approximately 1 year. Stents are removed after 1 year unless the tail of pancreas depends on stenting for duct drainage as determined by ERCP or MRCP. If MRCP identifies a disconnected duct in the tail of the pancreas, transenteric stents can be left in place indefinitely to facilitate drainage.
wire to enlarge the cyst-enterostomy. An opening of a minimum of 1 cm is preferable and is created by orienting the sphincterotome in multiple directions. A radial expansion balloon can be used to dilate the cyst-enterostomy in cases where there is bleeding from the mucosa. The pseudocyst is then drained with one to two 10 French stents, side by side (Fig. 2A to G). Double stenting is more effective than a single stent because the pseudocyst can drain alongside the stents as well as through them. Single stents can be encased by gastric mucosa and induce infection when their lumen becomes occluded. Since these stents remain in place long term, drainage alongside the stent may be more important than drainage through the stent, thus supporting the use of double stenting. The University of Louisville experience routinely dictates leaving stents in place for approximately 1 year. Stents are removed after 1 year unless the tail of pancreas depends on stenting for duct drainage as determined by ERCP or MRCP. If MRCP identifies a disconnected duct in the tail of the pancreas, transenteric stents can be left in place indefinitely to facilitate drainage.
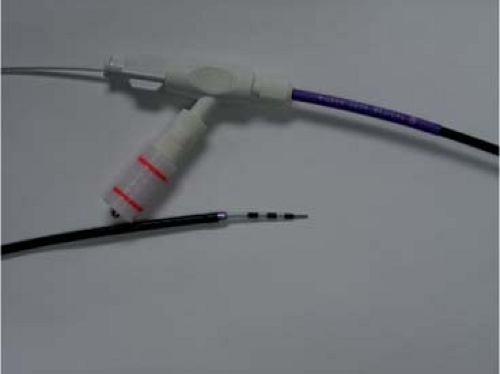
The most current techniques in endoscopic transmural drainage have introduced the use of the Cremer cystotome, first described in 1990, which combines a removable electrocautery needle knife with a circumferential 10 French electrocautery ring to allow for cyst puncture, guide wire passage, and cyst gastrostomy enlargement using a single catheter (Fig. 3). The cystotome facilitates difficult cyst access since no catheter exchange is necessary and the guide wire can be passed directly through the cutting device. This cystotome allows for rapid advancement of a guide wire into the cyst cavity when the visual field is obscured by fluid. Guide wire placement into the cyst cavity before catheter removal facilitates rapid stent deployment. Other advantages include the ability to perform cystography, and the small cauterized cyst-enterostomy prevents hemorrhagic complications. This is currently the preferred technique at the University of Louisville.
Eus-Guided Transmural Pseudocyst Drainage
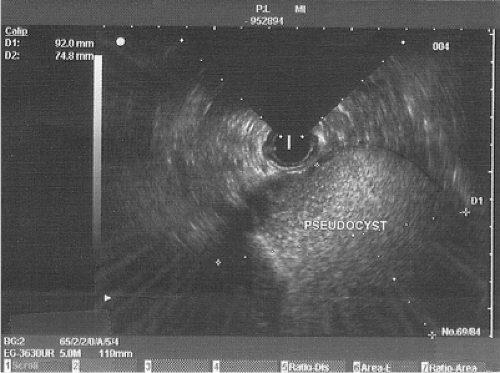
A second innovation that has allowed for more effective drainage of pancreatic pseudocysts and prevention of hemorrhagic complications is the use of EUS guidance. EUS is especially attractive in patients who have a small window of entry into the pseudocyst determined by CT, in patients in whom there is no enteric indentation, when the patient has a coagulopathy or thrombocytopenia, or where there are varices between the enteric lumen and pseudocyst. Several studies have demonstrated that EUS has a superior safety profile when compared with esophagogastroduodenoscopy EGD-directed transmural drainage. EUS enables identification of collateral vasculature, providing a safe window for transmural drainage and thus reducing hemorrhagic complications. A prospective randomized
trial performed by Varadarajulu (2008) demonstrated significantly greater technical success for EUS-guided drainage (100%) than for EGD-directed transmural drainage (33%), adjusting for luminal compression by the pseudocyst. This study stated that luminal compression may be related to the location of the pseudocyst, while other studies have found compression is related to pseudocyst size. Park (2009) addressed the issue of luminal compression and technical success rates in a prospective randomized trial. This trial demonstrated a success rate of 94% for EUS-guided drainage compared with 72% for EGD-guided drainage. Patients who failed EGD-guided drainage were found to have no luminal compression by EGD. Comparison of long-term results between the two techniques demonstrated no significant difference in clinical outcomes or complication rates. Overall, EUS-guided pseudocyst drainage is best suited for patients who lack significant enteric luminal compression and who have multiple risk factors for procedure-related complications (Fig. 4).
trial performed by Varadarajulu (2008) demonstrated significantly greater technical success for EUS-guided drainage (100%) than for EGD-directed transmural drainage (33%), adjusting for luminal compression by the pseudocyst. This study stated that luminal compression may be related to the location of the pseudocyst, while other studies have found compression is related to pseudocyst size. Park (2009) addressed the issue of luminal compression and technical success rates in a prospective randomized trial. This trial demonstrated a success rate of 94% for EUS-guided drainage compared with 72% for EGD-guided drainage. Patients who failed EGD-guided drainage were found to have no luminal compression by EGD. Comparison of long-term results between the two techniques demonstrated no significant difference in clinical outcomes or complication rates. Overall, EUS-guided pseudocyst drainage is best suited for patients who lack significant enteric luminal compression and who have multiple risk factors for procedure-related complications (Fig. 4).
Peroral Endoscopic Stapler Application for Cyst-Gastrostomy
A third innovation addresses both the adequacy of the cyst enterostomy and the potential for hemorrhage from the stomach wall by using a peroral endoscopic stapler. Romanelli (2008) has demonstrated the effective use of a peroral computer-mediated stapler device (SurgAssist; Power Medical Interventions, Langhorne, PA) in two patients who had complete pancreatic pseudocyst resolution at 6 weeks with no complications (Fig. 5). The procedural description includes introduction of a standard gastroscope into the stomach and creation of transmural gastric access either under direct vision or under EUS guidance. An overtube (20 mm) is then advanced over the gastroscope into the stomach. The peroral endoscopic stapling device is inserted into the stomach through the overtube. The overtube is then removed, a gastroscope is inserted alongside the stapler, and a snare is used to grasp one of the open jaws of the stapler in order to drag the device to the cyst gastrostomy orifice. The anvil is inserted into the pseudocyst cavity with the staple cartridge in the stomach lumen. Two applications of the stapler create a “fish-mouthed” stapled cyst-gastrostomy between 7.5 and 8.5 cm long. This device has been demonstrated to be both safe and effective, allowing extensive drainage of necrotic debris from complex pseudocysts.
Endoscopic Drainage of Pancreatic Abscesses
Infection and abscess formation in a pseudocyst cavity has previously been associated with failure of endoscopic therapy. Abscess formation with a minimum of pancreatic necrosis has recently been reported to be successfully drained using endoscopic techniques by several studies. Pancreatic abscesses can be found in 2% to 5% of patients admitted to the hospital for pancreatitis with an incidence as high as 25% in severe pancreatitis. Surgical laparotomy has been associated with a mortality rate from 10% to 59% and a morbidity rate as high as 60% to 93%. Percutaneous drainage of these collections carries a rate of pancreatic fistula formation as high as 39%. The Louisville experience (35 patients) reported by Vitale (2009) demonstrated an 80% success rate in draining pancreatic abscesses (15-month follow-up) utilizing both transmural and transpapillary drainage techniques. Similar studies by Binmoeller (1993) and Venu (2000) demonstrated success rates of 74% to 80% on long-term follow-up for abscess drainage. The Louisville experience demonstrated independent predictors for successful outcome including location of the cyst in the pancreatic head, insertion of multiple stents into the pseudocyst cavity, and drainage of the pseudocyst cavity for longer than 6 weeks. Moderate abscess debris was the primary independent predictor for failure of endoscopic drainage in this study. Further studies have demonstrated the feasibility of pancreatic debridement using endoscopic techniques to address this issue.
Direct Endoscopic Necrosectomy
Necrotic pancreatic tissue within the pseudocyst cavity has been identified as an independent predictor of the failure of endoscopic drainage techniques. The presence of extensive necrosis within a pancreatic fluid collection has been defined by several studies as WOPN (formerly called organized pancreatic necrosis). WOPN can be described as the evolution of acute necrosis into a partially encapsulated well-defined collection of pancreatic fluid and debris. Baron (2002) compared resolution of pancreatic fluid collections in a series of 138 patients, demonstrating a 92% rate for chronic pseudocysts compared with 74% in acute pseudocysts and 72% in the setting of pancreatic necrosis. The inefficacy
of transmural endoscopic drainage in treating pancreatic necrosis has led to the development of direct endoscopic necrosectomy. Endoscopic necrosectomy begins with endoscopic transmural access to a pancreatic fluid collection. Necrotic pancreatic tissue identified within the cavity is then removed using a combination of several devices including 10 French irrigation probes, 15-mm stone retrieval balloons, Roth retrieval baskets, stone retrieval baskets, tripod retrieval forceps, rat-toothed and pelican forceps. Stenting with two pigtail stents is then used to maintain the patency of the transmural access site to allow further drainage of fluid and necrotic debris. Voermans (2007) described serial transmural pancreatic debridement under direct vision including nasocystic catheter irrigation with a long-term (16-month) success rate of 93%. Seifert (2010) described the largest series to date (93 patients) of endoscopic pancreatic necrosis debridement (mean 6 interventions) demonstrating initial clinical success of 80% with a 26% complication rate and a 7.5% mortality rate at 30 days. Long-term follow-up of 43 months in this study demonstrated an overall success rate of 84%, with 10% requiring further endoscopic therapy and 4% receiving surgical treatment. Gardener (2009) demonstrated successful resolution of WOPN in 88% of patients who underwent direct endoscopic necrosectomy compared with resolution in 45% of patients who received standard transmural drainage.
of transmural endoscopic drainage in treating pancreatic necrosis has led to the development of direct endoscopic necrosectomy. Endoscopic necrosectomy begins with endoscopic transmural access to a pancreatic fluid collection. Necrotic pancreatic tissue identified within the cavity is then removed using a combination of several devices including 10 French irrigation probes, 15-mm stone retrieval balloons, Roth retrieval baskets, stone retrieval baskets, tripod retrieval forceps, rat-toothed and pelican forceps. Stenting with two pigtail stents is then used to maintain the patency of the transmural access site to allow further drainage of fluid and necrotic debris. Voermans (2007) described serial transmural pancreatic debridement under direct vision including nasocystic catheter irrigation with a long-term (16-month) success rate of 93%. Seifert (2010) described the largest series to date (93 patients) of endoscopic pancreatic necrosis debridement (mean 6 interventions) demonstrating initial clinical success of 80% with a 26% complication rate and a 7.5% mortality rate at 30 days. Long-term follow-up of 43 months in this study demonstrated an overall success rate of 84%, with 10% requiring further endoscopic therapy and 4% receiving surgical treatment. Gardener (2009) demonstrated successful resolution of WOPN in 88% of patients who underwent direct endoscopic necrosectomy compared with resolution in 45% of patients who received standard transmural drainage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree